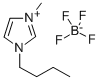Synthesis and Application of 1-Butyl-3-methylimidazolium tetrafluoroborate
Aug 22,2022
General description
1-Butyl-3-methylimidazolium tetrafluoroborate as a bulk solvent phase offers new opportunities for recyclable alternatives to conventional organic solvents for biocatalytic production of commercially important chemicals, including non- Symmetrical synthesis and in some cases increased product formation rate and enantioselectivity. 1-Butyl-3-methylimidazolium tetrafluoroborate has been used as an enzyme coating for the development of recyclable heterogeneous biocatalysts in continuous-phase systems employing ScCO2 as enzyme immobilization in biphasic systems The carrier and as part of the supporting liquid membrane that continuously separates reactants and products in an enzyme-catalyzed reaction.
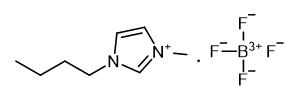
Fig. 1 The structure of 1-Butyl-3-methylimidazolium tetrafluoroborate.
Synthetic routes
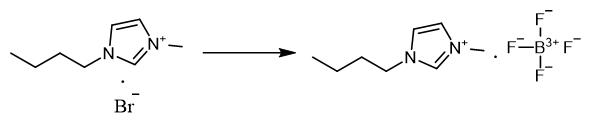
Fig. 2 The synthetic method 1 of 1-Butyl-3-methylimidazolium tetrafluoroborate.
Add 6.57 g (0.03 mol) [BMIM]Br and 3.29 g (0.03 mol) NaBF4 to the single mouth flask with a definite amount of acetone as solvent, for 10 hours at 40°C under vigorous stirring. Filter the reaction mixture and distil under vacuum. Add dichloromethane to the residue of [BMIM]Br and NaBF4 to obtain white solids precipitated. Separate the solid precipitate by filtration. Dry the product vacuum in an oven at 80°C for 2 hours to remove the traces of dichloromethane [1].
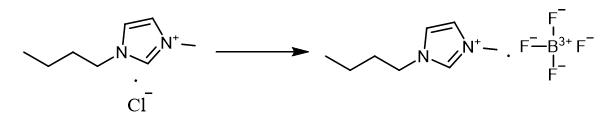
Fig. 3 The synthetic method 2 of 1-Butyl-3-methylimidazolium tetrafluoroborate.
Dissolve butylmethylimidazolium chloride (14.52 g, 0.083 mol) in acetone. Add sodium tetrafluoroborate (23.89 g, 0.083 mol) to the solution. Stir the reaction mixture for two days. Evaporate the solvent. Dissolve ionic liquid in dichloromethane. Evaporate the solvent from mixture. Dry the residue in the vacuum to obtain butylmethylimidazolium tetrafluoroborate. 1H NMR (200 MHz, DMSO-d6), δ/ppm: 0.8 (t, 3H, CH2-CH3), 1.2 (m, 2H, CH2-CH2-CH3), 1.7 (m, 2H, CH2-CH2-CH2), 3.8 (s, 3H, CH3-N), 4. 1 (t, 2H, N-CH2-CH2), 7.6 (d, 2H, N-CH-CH-N), 8.9 (d, 1H, N-CH-N) [2].
Application
Ionic Liquids
Composite silica xerogels were prepared via acid catalysed sol-gel route using tetraethoxysilan (TEOS) as silica precursor, and 1-butyl-3-methylimidazolium tetrafluoroborate [BMIM][BF4] or 1-butyl-3-methylimidazolium chloride [BMIM][Cl] ionic liquids, used simultaneously as co-solvents, catalysts and pore templates, at various IL-to-silica ratios. Morphology of the xerogels prepared using the different IL templating agents were investigated using scanning electron microscopy (SEM), nitrogen sorption and small angle neutron scattering (SANS). The thermal behavior of the composites was analyzed by thermal gravimetry, whereas the compositions were checked by infrared spectroscopy and EDX. The differences in the morphology and thermal behavior of the composites due to the different IL additives were revealed [3].
Artificial neural network (ANN) has been used to estimate the solubility of sulfur dioxide in different ionic liquids. The studied ionic liquids are consisting of 1-butyl-3-methylimidazolium acetate [BMIM][Ac], 1-n-butyl-3-methylimidazolium methylsulfate [BMIM][MeSO4], 1-Butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]), 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]), 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide [BMIM][Tf2N], 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]), 1-Hexyl-3-methylimidazolium tetrafluoroborate ([HMIM][BF4]) and 1-Hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [HMIM][Tf2N]. The model has five inputs including temperature, pressure, critical temperature, critical pressure and acentric factor. The network has been trained with about 70% of data sets and tested with other 30% of data points. The most accurate network has one hidden layer with 27 neurons. The results of this study show that the ANN model is successful in estimation of the solubility of sulfur dioxide in ionic liquids [4].
Physical and electrochemical properties
The density, viscosity, refractive index, heat capacity, heat of dilution, ionic conductivity, and electrochemical stability of 1-butyl-3-methylimidazolium bromide ([bmim][Br]), 1-butyl-3-methylimidazolium iodide ([bmim][I]), and 1-butyl-3-methylimidazolium tetrafluoroborate ([bnum][BF4]) were measured at room temperature or over a temperature range of 293.2 to 323.2 K. The density and refractive index values of [bmim][I] appeared to be the highest among three ionic liquids (ILs). However, the experimental viscosity values of [bmim][Br] were higher than those of [bmim][BF4], while the heat capacities and heats of dilution of [bmim][BF4] were higher than those of [bmim][Br]. The cyclic voltammogram of [bmin][br] and [bmim][BF4] indicated electrochemical windows in the stability range from 2.7 V of [bmim][Br] to 4.7 V of [bmim][BF4] [5].
1-Butyl-3-methylimidazoliumtetrafluoroborate ([BMIm][BF4]) + 2,2,2-trifluoroethanol (TFE) and 1-butyl-3-methylimidazolium bromide ([BMIm][Br])+ TFE mixtures exhibit properties which render them suitable as candidates for working fluids in absorption heat pumps or chillers. Refractive indices and heat capacities of each pure ionic liquid were investigated in the temperature range of 298.2-323.2 K. Vapor pressures were measured by using the boiling point method in the concentration range of 40.0-90.0 mass% of ionic liquid and were correlated with an Antoine-type equation. The average absolute deviations between experimental and calculated values were 0.6% for the [BMIm][BF4]+ TFE and 0.4% for the [BMIm] [Br]+ TFE system, respectively [6].
References
[1] Gill C H, Kulkarni M V, Jadhav C K, et al. Efficient and environmentally sustainable domino protocol for the synthesis of diversified dispiroheterocycles using 1-Butyl-3-methylimidazolium bromide [bmim] Br[J]. Chemical Review and Letters, 2021, 4(3): 153-163.
[2] Leu M, Campbell P, Mudring AV. Synthesis of luminescent semiconductor nanoparticles in ionic liquids -the importance of the ionic liquid in the formation of quantum dots [J]. Green Chemistry Letters and Reviews, 2021, 14(1), 128-136.
[3] Putz A M, Len A, Trif L, et al. Imidazolium Ionic Liquids as Designer Solvents Confined in Silica Nanopores[J]. Gels, 2022, 8(6): 388.
[4] Bahmani A R, Sabzi F, Bahmani M. Prediction of solubility of sulfur dioxide in ionic liquids using artificial neural network[J]. Journal of Molecular Liquids, 2015, 211: 395-400.
[5] Kim K S, Shin B K, Lee H. Physical and electrochemical properties of 1-butyl-3-methylimidazolium bromide, 1-butyl-3-methylimidazolium iodide, and 1-butyl-3-methylimidazolium tetrafluoroborate[J]. Korean Journal of Chemical Engineering, 2004, 21(5): 1010-1014.
[6] Kim K S, Shin B K, Lee H, et al. Refractive index and heat capacity of 1-butyl-3-methylimidazolium bromide and 1-butyl-3-methylimidazolium tetrafluoroborate, and vapor pressure of binary systems for 1-butyl-3
- Related articles
- Related Qustion
1-Butyl-3-methylimidazolium tetrafluoroborate
174501-65-6You may like
1-Butyl-3-methylimidazolium tetrafluoroborate manufacturers
- 1-Butyl-3-methylimidazolium tetrafluoroborate
-

- $0.00 / 25KG
- 2025-12-11
- CAS:174501-65-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 1-Butyl-3-methylimidazolium tetrafluoroborate
-

- $18.00 / 10KG
- 2025-10-13
- CAS:174501-65-6
- Min. Order: 0.01KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- 1-Butyl-3-methylimidazolium tetrafluoroborate
-

- $0.00 / 1KG
- 2025-06-27
- CAS:174501-65-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg