Synthesis and application of hexafluoro dianhydride (6FDA)
Aug 26,2022
General description
Hexafluoro dianhydride (i.e. 4,4 '- (hexafluoroisopropylidene) di phthalic anhydride, 6FDA)) is one of the six most widely used dianhydride monomers, and is also the most widely used dianhydride monomer in colorless transparent polyimides[1-2]. The glass transition temperature of polyimide synthesized from hexafluorodianhydride is usually above 300 ° C, and the mechanical and electrical properties are well balanced. So far, it is still the most representative fluorinated polyimide[3].
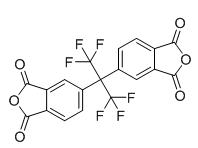
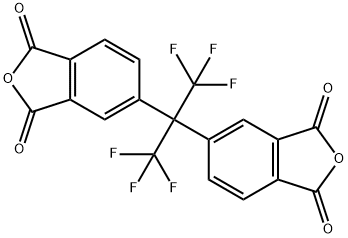
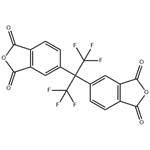
Fig. 1 The structure of hexafluoro dianhydride.
Synthesis
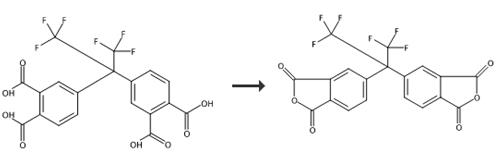
Fig. 2 The synthetic route of hexafluoro dianhydride[4-5].
(1)2,2-bis(3,4-dimethylphenyl)hexafluoropropane is oxidized to 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane. 2,2-bis(3,4-dimethylphenyl)hexafluoropropane and 35% HNO3 are fed into a continuous-flow tubular reactor. The tube diameter is 100 mm and length is 12 m. The temperature of the mixture within the reactor is increased to between 200 °C and 240 °C. The volumetric flow in the reactor is set to 5.9 liters/minute. Nitric oxides are formed as a reaction by-product and are recycled to form HNO3. The effluent from the reactor is collected, depressurized, and cooled to room temperature. HNO3 is removed from the effluent. The desired product 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane precipitates within the effluent and is separated by centrifugation and washed with water. The purity of the product is 97% 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane as determined by high pressure liquid chromatography (HPLC). Formation of 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane anhydride The anhydride formation reaction is represented by the following: The 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane is subsequently converted to 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane anhydride. The 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane (water moist) is added to a hydrocarbon fraction having a boiling point of between 180 °C and 200 °C. Residual water is distilled off. The mixture is further heated to 160 °C and reacted for 3 hours. The mixture is cooled to 20 °C, and the 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane anhydride is separated via centrifugation, washed with acetic acid, and dried. The purity of the product is 97% 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane anhydride (HPLC). Recrystallization with acetic acid/acetanhydride/activated carbon yields a product 2,2-bis(3,4-dicarboxyphenyl)hexafluoropropane anhydride of >99% purity (HPLC).
(2)The ion exchange water 0 g was added to 249 g of mixed solution (2 wt% of water content) obtained in Example 1 (10.9 wt% of water content), it was stirred at 50 °C for 0.5 hour for homogeneous solution, 50 ml of cation exchange resin (Mitsubishi Chemical diagram ion RCP-160M) was added and stirred at 50 °C for 1 hour. After filtering off the resin, the solvent from the mother liquor and 0 g of water were distilled off under reduced pressure, acetic anhydride 68.0 g [(stoichiometric amount of 2,2-bis-(3,4-dicarboxyphenyl)-hexafluoropropane + amount of water x1.5 times] was added, stirred at 100 °C for 3 hours. The generated crystals were filtered after cooling the solution, dried at 100 °C under reduced pressure for 2 hours and 40.5 g (91.0% of yield) of almost white 2,2-bis-(3,4-dicarboxyphenyl)-hexafluoropropanedianhydride was obtained. The purity by HPLC analysis of this prodcut was 99.6% and by atomic absorption analysis, the content of cobalt and manganese was below 1 ppm each.
Application
Hexafluoric anhydride (6FDA) is an organic synthesis intermediate and pharmaceutical intermediate, which can be used in laboratory research and development processes and chemical and pharmaceutical synthesis processes, mainly as an electronic material polymer[6-8]. In terms of membrane materials, 6FDA–4,4′-(hexafluoroisopropylidene) dianiline (6FpDA) (synthesized from 6FDA moiety and 6FpDA moiety) has attracted much attention recently among the glassy polymers[9]. For instance, it was identified as a very promising material for olefin/paraffin mixture separation. A comparison of diffusion coefficients of O2 and N2 in 6FDA–6FpDA determined by transient sorption/permeation and steady-state sorption/permeation was reported. The permeability and solubility of CO2 and CH4 for 6FDA–6FpDA were also tested under different conditioning protocols to investigate the conditioning and plasticization responses. In addition, the permeability, solubility and physical properties of 6FDA–6FpDA for He, O2, N2, CH4 and CO2 were compared with its five polyimide isomers to reveal the effects of structural modifications[10-11]. The temperature influence on 6FDA–6FpDA was also examined and revealed that it is an outstanding material candidate for use in novel elevated temperature applications. Hollow fiber membranes fabricated from 6FDA–6FpDA have as well been developed for gas separation[12].
Extensive research efforts have been carried out on polymeric material selection for natural gas separation[13-14]. Among those, the family of 6FDA (2,2-bis(3,4-dicarboxylphenyl)hexafluoropropane dianhydride) based polyimides appears to be a promising candidate due to its excellent gas separation performance and mechanical properties. For instance, 6FDA-2,6-DAT (poly(2,6-toluene-2,2-bis(3,4-dicarboxylphenyl)hexafluoropropane diimide)), was reported to possess excellent intrinsic gas separation properties with a CO2/CH4 selectivity of around 45 and a CO2 permeability of 40 barrer at 35 °C and 1013.25 kPa (10 atm). In addition, the 6FDA-2,6-DAT asymmetric composite hollow fibers have been successfully prepared by an environmental-friendly fabrication technology in our laboratory. The stabilized hollow fibers possess good separation performances with a CO2/CH4 selectivity of 40 and a CO2 permeance of 59×10?6 cm3(STP)/cm2 s cmHg (59 GPU) under mixed gas tests. However, these hollow fibers are prone to be plasticized by CO2 even at a very low pressure of 344.7 kPa (50 psi)[15-17]. In order to use 6FDA-2,6-DAT hollow fibers in natural gas separation, one must find ways to enhance their resistant characteristics to CO2-induced plasticization.
Storage
Storage precautions of hexafluoro dianhydride (6FDA) are described as follows: Store in a cool and ventilated warehouse. Keep away from fire and heat source. Packing should be sealed and out of contact with air[18]. It should be stored separately from oxidants, acids and edible chemicals, and must not be mixed. Explosion-proof lighting and ventilation facilities are adopted. Do not use mechanical equipment and tools that may cause sparks. The storage area shall be equipped with leakage emergency treatment equipment and suitable storage materials.
Reference
[1] A. Balasubramanian, M. Gunasekaran, T. Kannan, Photo crosslinked stilbene-containing sulfonated polyimide membranes as proton exchange membranes in fuel cell, Eur. Polym. J. 176 (2022) 111418.
[2] S.C. Ganguly, J.G. Matisons, Low voltage scanning electron microscopy of a surface modified teflon membrane and thermal analysis studies of several anhydride modified Nafion membranes, Abstracts of Papers, 2
- Related articles
- Related Qustion
- Unveiling the Versatility of 4,4'-(Hexafluoroisopropylidene) diphthalic anhydride Jul 23, 2024
4,4'-(Hexafluoroisopropylidene)diphthalic Anhydride stands as a noteworthy compound, renowned for its unique structural and functional properties.
You may like
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride manufacturers
- 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
-
- 2025-12-13
- CAS:1107-00-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
-

- $0.00 / 1KG
- 2025-12-13
- CAS:1107-00-2
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
-

- $5.00 / 25kg
- 2025-12-12
- CAS:1107-00-2
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 100mt/year