Thorium dioxide: a fuel in nuclear reactors
May 20,2024
Introduction
Thorium dioxide (ThO2) is a ceramic material with industrial applications owing to its high melting point (3300 °C) and strong insulating properties. Thorium has attracted much attention as an alternative nuclear fuel to uranium. Thorium is assumed to be three to four times more abundant in nature than uranium. Thorium dioxide is chemically more stable than uranium dioxide. Regarding economy and safety, thorium fuel is expected to be a candidate fuel for next-generation nuclear reactors[1].
As a fuel in nuclear reactors
Thorium dioxide is of particular research interest owing to its applications in nuclear reactors for power generation[2]. Currently, reactors capable of using ThO2 fuels include the popular pressurized water reactors (PWRs), although most PWRs presently use uranium-based fuels. Several of the new reactor designs, the so-called Generation IV reactors, are also capable of using thorium-based fuels. While thorium fuels can take the form of either oxide or fluoride salt, current reactor technology is heavily biased in favor of mixed oxide reactors, where uranium and plutonium oxides are used as mixed oxide fuels (MOX). ThO2, PuO2, and UO2 all share the fluorite structure (space group 225), ThO2 is a good candidate for MOX fuel reactors and offers many safety benefits. As thorium is not a fissile element, a sustained nuclear decay reaction can only occur in the presence of a neutron source. Both plutonium and uranium produce neutrons and thus are suitable in thorium-based fuels. Once the neutron source is depleted, thorium ceases to undergo further radioactive decay, thereby forestalling any reactor meltdowns. Additionally, thorium dioxide is considered proliferation-resistant due to the extreme difficulty of isolating any potential weapons-grade nuclear material from its waste products. Reprocessed waste uranium or plutonium from conventional MOX reactors can be used, thus reducing nuclear waste stores.
Structure
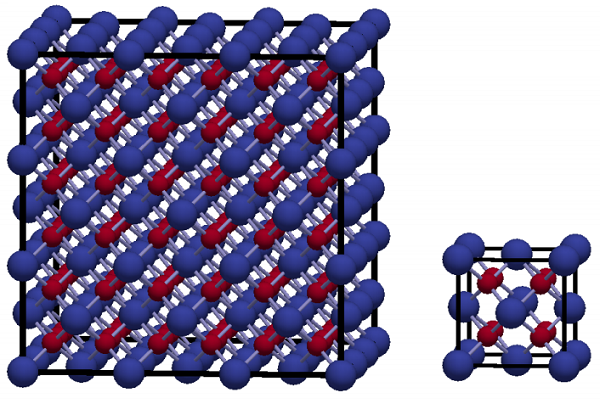
As shown above, thorium dioxide has a cubic crystal structure with a lattice constant of 5.592 Å. Its structure is equivalent to fluorite, whose space group is Fm3m. Th and O atoms occupy the Wyckoff positions 4a and 8c, respectively. Its melting point is 3651 K, which is higher than that of uranium dioxide (3120 K).
References
[1] Hiroki Nakamura, Masahiko Machida. “High-temperature properties of thorium dioxide: A first-principles molecular dynamics study.” Journal of Nuclear Materials 478 (2016): Pages 56-60.
[2] Ashley E. Shields , Nora H. de Leeuw, David Santos-Carballal . “A density functional theory study of uranium-doped thoria and uranium adatoms on the major surfaces of thorium dioxide.” Journal of Nuclear Materials 473 (2016): Pages 99-111.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringThe zirconium silicide intermetallic compounds with the 16H crystal structure are good materials for high temperature structural applications, because of their high melting point and low density.....
May 20,2024APIThorium dioxide
1314-20-1You may like
- Thorium dioxide
-

- 2025-12-18
- CAS:1314-20-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Thorium dioxide
-

- $0.00 / 1KG
- 2024-09-03
- CAS:1314-20-1
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 10000KG
- thorium oxide
-

- $189.00 / 1kg
- 2024-06-11
- CAS:1314-20-1
- Min. Order: 100kg
- Purity: 99.5%
- Supply Ability: 20T






