Use and Safety of Grepafloxacin
Mar 25,2022
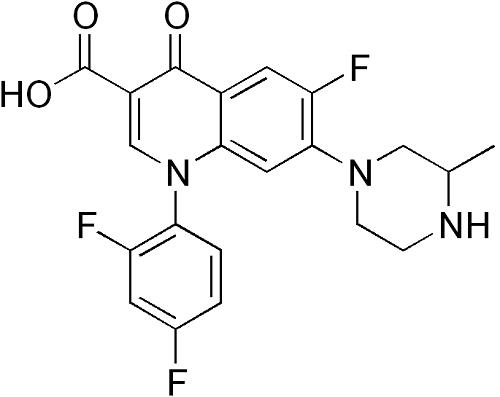
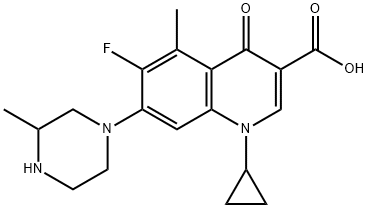
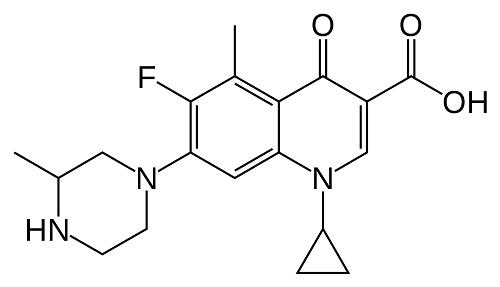
Grepafloxacin (OPC-17116) is a broad-spectrum fluoroquinolone with the chemical structure (+)-1-cyclopropyl-6-fluoro-1,4-dihydro-5- methyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolone carboxylic acid hydrochloride. It was first marketed in August 1997 and became available in over 30 countries. It was withdrawn in October 1999 following several reports of grepafloxacin-induced ventricular dysrhythmias, presumed secondary to QT-interval prolongation (FDA, 1999b).
Uses
In comparison with earlier fluoroquinolones, grepafloxacin has enhanced activity against Gram-positive cocci such as S. pneumoniae and some strains of methicillin-resistant S. aureus, but generally less activity than ciprofloxacin against many Gram-negative pathogens. The MIC90s of grepafloxacin against Enterobacteriaceae, S. aureus, and S. pneumoniae are r0.25, r0.125, and r0.5 mg/ml, respectively. Grepafloxacin retains activity against penicillin-resistant S. pneumoniae. Notably, grepafloxacin does not generally inhibit methicillin-resistant, ciprofloxacin- resistant strains of S. aureus (MIC90 W8 to 32mg/ml) or coagulase-negative staphylococci (MIC90W4 mg/ml). Grepafloxacin is less active against Pseudomonas aeruginosa (MIC90 2 to W8 mg/ml). Haemophilus influenzae, Moraxella catarrhalis, and Neisseria gonorrhoeae are susceptible with MIC90s of r0.25, r0.03, and 0.004 mg/ml, respectively. Against atypical respiratory pathogens, grepafloxacin has excellent in vitro activity with MIC90s to Chlamydophila pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae being 0.12, o0.5, and 0.5 mg/ml, respectively.
Activity
Grepafloxacin has intermediate activity against anaerobes, generally having MICs one or two doubling dilutions lower than those obtained with ciprofloxacin. Against a broad range of anaerobes the MIC50 and MIC90 of grepafloxacin are 2 and 16 mg/ml, respectively. Using a breakpoint of 2 mg/ml, grepafloxacin inhibits 83% of Bacteroides fragilis spp., compared with 6% for ciprofloxacin. However, against other B. fragilis group anaerobes, grepafloxacin inhibits only 39% of strains. Grepafloxacin is 2- to 20-fold more active in vitro than norfloxacin, ofloxacin, tosufloxacin, and ciprofloxacin against a wide variety of common and rare Nocardia species, with an MIC90 of 0.6 to 20 mg/ml. The clinical efficacy of grepafloxacin in the treatment of Nocardia infections is uncertain.
Metabolism
Grepafloxacin appears to be rapidly absorbed from the gastrointestinal tract. After a single 400-mg oral dose of grepafloxacin given to six healthy male volunteers, the mean peak plasma concentration was 1.5 mg/ml a mean of 2.0 h post dose. The mean elimination halflife in plasma is 5.2 h. Plasma and exudate concentrations generally exceed 0.5 mg/ml for more than 10–12 h after a single oral dose. Tissue penetration is excellent, with high drug levels measured in respiratory bronchial mucosa, biliary tissues, female genital tissue, and prostatic tissue following oral dosing. Compared with ciprofloxacin, excretion of grepafloxacin in urine is low, with 8.3% of the total dose recoverable from urine. Elimination of grepafloxacin is predominantly via hepatic metabolism, and in subjects with hepatic impairment, peak plasma concentrations and renal excretion are significantly increased compared with controls. Grepafloxacin does not interact with warfarin;however, theophylline plasma levels double when it is co-administered.
Safety and tolerability
In preclinical studies and in postmarketing surveillance, the most common adverse events associated with grepafloxacin were gastrointestinal, particularly nausea and taste perversion (in up to 15% and 17% of patients, respectively, taking 600 mg daily). Grepafloxacin was withdrawn commercially in October 1999, 24 months after its initial release, owing to cardiac complications presumed secondary to drug-induced QTc-interval prolongation. In postmarketing surveillance, the use of grepafloxacin was"possibly"associated with seven sudden cardiac deaths and several cases of torsade de pointes (TP) – a malignant polymorphic ventricular tachyarrhythmia.
The mechanism leading to QT prolongation is thought to be inhibition of the critical human ether-a-go-go (HERG) K+ channel that regulates the rapid component of the delayed rectifier current (IKr) in cardiac tissue. All fluoroquinolones tested have been found to block this channel but with widely different potencies. The IC50 for HERG K+ channel inhibition for sparfloxacin and grepafloxacin has been measured at 18 and 50 mM, respectively, whilst for moxifloxacin and ciprofloxacin, the IC50 values are considerably higher (129 and 966 mM, respectively).
- Related articles
- Related Qustion
Temafloxacin (6-fluoro-7-piperazino-4-quinolone)was discontinued in June 1992, only five months after gaining marketing approval. This was due to a high rate of reported adverse reactions, including several deaths.....
Mar 25,2022APIIsoniazid, isonicotinic acid hydrazide (INAH or INH), was discovered independently in 1952 at both Squibb and Roche Laboratories. Animal studies showed that isoniazid was a very potent antituberculosis drug, and subsequent clinical trials c....
Mar 25,2022APIGrepafloxacin
119914-60-2You may like
Grepafloxacin manufacturers
- Grepafloxacin USP/EP/BP
-
- $1.10 / 1g
- 2025-11-18
- CAS:119914-60-2
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons min
- Grepafloxacin
-

- $1.00 / 1g
- 2019-12-26
- CAS:119914-60-2
- Min. Order: 1g
- Purity: ≥98%
- Supply Ability: g/kg/Ton