Uses of Calcium chloride
Dec 29,2021
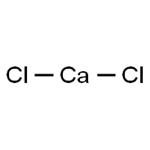
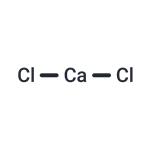
Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl2. It is a white colored crystalline solid at room temperature, and it is highly soluble in water. It can be created by neutralising hydrochloric acid with calcium hydroxide.
Calcium Chloride is a mineral indicated in the immediate treatment of hypocalcemic tetany (abnormally low levels of calcium in the body that cause muscle spasm). Calcium chloride injection is also used in cardiac resuscitation, arrhythmias, hypermagnesemia, calcium channel blocker overdose, and beta-blocker overdose.

Uses
De-icing and freezing-point depression
By depressing the freezing point of water, calcium chloride is used to prevent ice formation and is used to de-ice. This application consumes the greatest amount of calcium chloride. Calcium chloride is relatively harmless to plants and soil. As a deicing agent, it is much more effective at lower temperatures than sodium chloride. When distributed for this use, it usually takes the form of small, white spheres a few millimeters in diameter, called prills. Solutions of calcium chloride can prevent freezing at temperatures as low as −52 °C (−62 °F), making it ideal for filling agricultural implement tires as a liquid ballast, aiding traction in cold climates.
It is also used in domestic and industrial chemical air dehumidifiers.
Road surfacing
The second largest application of calcium chloride exploits its hygroscopic nature and the tackiness of its hydrates; calcium chloride is highly hygroscopic and its hydration is an exothermic reaction. A concentrated solution keeps a liquid layer on the surface of dirt roads, which suppresses the formation of dust. It keeps the finer dust particles on the road, providing a cushioning layer. If these are allowed to blow away, the large aggregate begins to shift around and the road breaks down. Using calcium chloride reduces the need for grading by as much as 50% and the need for fill-in materials as much as 80%.
Food
The average intake of calcium chloride as food additives has been estimated to be 160–345 mg/day.Calcium chloride is permitted as a food additive in the European Union for use as a sequestrant and firming agent with the E number E509. It is considered as generally recognized as safe (GRAS) by the U.S. Food and Drug Administration.Its use in organic crop production is generally prohibited under the US National Organic Program.
- Related articles
- Related Qustion
- Properties, Preparation and Ionic Structure of Calcium Chloride Feb 7, 2024
Calcium Chloride is an ionic compound composed of chlorine and calcium. At room temperature, it appears as a crystalline solid with a white color. The compound of CaCl2 is electrically neutral.
- Preparation and Flame Test of Calcium Chloride Jan 26, 2024
Calcium chloride is an ionic compound,It is odourless and has a very high enthalpy change of solution. This compound is widely used for dust control and de-icing and displays an orange-red flame when burning.
- Calcium Chloride Solution Nov 17, 2022
The passage introduces the Calcium Chloride Solution.
Sodium polyphosphate is a preservative against bacteria, molds, and yeasts. When used in high concentrations, it can cause skin irritation. Polyphosphates are sequestering and deflocculating agents, primarily in water treatment.....
Dec 29,2021Inorganic saltsCesium was discovered in 1860 by Robert Bunsen and Gustav Kirchoff. It is used in the most accurate atomic clocks. Cesium melts at 28.41°C (just below body temperature) and occurs in Earth’s crust at 2.6 ppm. Cesium is the rarest of the nat....
Dec 30,2021Inorganic chemistryCalcium chloride
10043-52-4You may like
Calcium chloride manufacturers
- Calcium chloride
-
- 2025-12-15
- CAS:10043-52-4
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Calcium chloride
-

- $50.00 / 25kg
- 2025-12-15
- CAS:10043-52-4
- Min. Order: 1000kg
- Purity: 0.94
- Supply Ability: 3000 MT per year
- Calcium chloride
-
- $29.00 / 100g
- 2025-12-12
- CAS:10043-52-4
- Min. Order:
- Purity:
- Supply Ability: 10g






