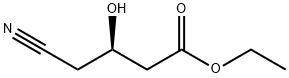
What is Ethyl (R)-(-)-4-cyano-3-hydroxybutyate?
Feb 25,2020
Hydroxyglutaryl coenzyme A (HMG-CoA) reductase inhibitors are a new class of lipid-lowering drugs, and they have a market share of more than 10 billion US dollars in the current pharmaceutical field[1]. They can selectively inhibit the rate-limiting enzyme HMG-CoA reductase in cholesterol synthesis in the liver, reduce liver lipoprotein production, and increase the expression of low-density lipoprotein (LDL) cholesterol receptors, thereby reducing plasma cholesterol levels[2]. They can also significantly reduce very low-density lipoprotein (VLDL) and triglycerides, and increase anti-atherosclerotic high-density protein (HDL), preventing atherosclerosis and coronary heart disease.

Ethyl (R)-(-)-4-cyano-3-hydroxybutyate is an important intermediate for the synthesis of HMG-CoA reductase inhibitors[3]. If it is used as a raw material, synthetic atorvastatin is mainly used for the treatment of hypercholesterolemia and mixed hyperlipidemia, coronary heart disease and stroke. Sales amounted to $ 7.8 billion in 2008. Therefore, how to reduce the synthesis cost and study the new method for the preparation of ethyl R-(-)-4-cyano-3-hydroxybutyrate have been the focus of attention at home and abroad[4]. In the reported synthesis.The main methods are:
Starting from chiral 3-hydroxy-γ-butyrolactone, Ethyl (R)-(-)-4-cyano-3-hydroxybutyate was prepared through ring opening, esterification and cyanation[5].
Racemic epichlorohydrin was used as a raw material to produce ethyl R-(-)-4-cyano-3-hydroxybutyrate using nitrilase.
A chiral epichlorohydrin was used as a raw material to undergo cyanide ring opening and nitrile hydrolysis, and then esterify to synthesize ethyl R-(-)-4-cyano-3-hydroxybutyrate[6].
A strain was obtained by screening by Chartrain, etc., and the ethyl 4-chloroacetoacetate could be biotransformed into ethyl S-4-cyano-3-hydroxybutyrate, and then cyanated to Ethyl (R)-(-)-4-cyano-3-hydroxybutyate[7].
The fermentation method is not efficient and is still in the laboratory research stage; the product obtained by the asymmetric synthesis method is not high in optical purity; the chiral source conversion method requires multi-step synthesis, the yield is low, and there are many by-products.
References
[1] Ming-Jia Yang, Xiang-Jing Wang, Zhong-Yi Yang. Bioconversion of ethyl (R)-4-cyano-3-hydroxybutyate into (R)-ethyl-3-hydroxyglutarate via an indirect pathway byRhodococcus boritolerans[J]. 34(5):901-905.
[2] Hua-Ping Dong, Zhi-Qiang Liu, Yu-Guo Zheng. Novel biosynthesis of (R)-ethyl-3-hydroxyglutarate with (R)-enantioselective hydrolysis of racemic ethyl 4-cyano-3-hydroxybutyate byRhodococcus erythropolis[J]. 87(4):1335-1345.
[3] Yao P, Li J, Yuan J, et al. Enzymatic Synthesis of a Key Intermediate for Rosuvastatin by Nitrilase‐Catalyzed Hydrolysis of Ethyl (R)‐4‐Cyano‐3‐hydroxybutyate at High Substrate Concentration[J]. 2015, 7(2):271-275.
[4] Burk, Mark J, Desantis, Grace, Morgan, Brian, et al. Processes for making [R]-ethyl 4-cyano-3 hydroxybutyric acid[J].
[5] Jiang, Chengjun, Hong, Huabin. ChemInform Abstract: A New Practical Synthesis of Ethyl (R)-(-)-4-Cyano-3-hydroxybutyrate (VII) from (S)-3-Chloro-1,2-propanediol (I).[J]. Cheminform, 44(1):no-no.
- Related articles
- Related Qustion
Strontium zirconate as mixed metal oxides with different structural characteristics can be widely applied in the field of electronic ceramics and refractory materials of high melting temperatures.....
Feb 25,2020Inorganic chemistry2,2'-Azobis(2-methylpropionitrile) is used as an initiator in polymer radical polymerization because its molecules can easily undergo split reactions and form molecules with high activation energy.....
Feb 25,2020Organic ChemistryEthyl (R)-(-)-4-cyano-3-hydroxybutyate
141942-85-0You may like
Ethyl (R)-(-)-4-cyano-3-hydroxybutyate manufacturers
- Ethyl (R)-(-)-4-cyano-3-hydroxybutyrate
-

- $5.00 / 200kg
- 2025-12-15
- CAS:141942-85-0
- Min. Order: 1kg
- Purity: ≥98%
- Supply Ability: 200mt/year
- Ethyl (R)-(-)-4-cyano-3-hydroxybutyate
-

- $50.00 / 1kg
- 2025-09-26
- CAS:141942-85-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20Tons
- (R)-4-cyano-3-hydroxybutyric acid ethyl ester
-
- $0.00 / 1KG
- 2025-07-08
- CAS:141942-85-0
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1000