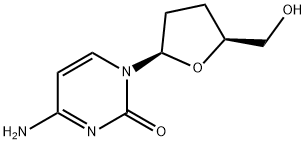
What is Zalcitabine?
Apr 14,2022
The synthetic pyrimidine nucleoside analog zalcitabine (2u,3u-dideoxycytidine, also known as ddC) was the third antiretroviral agent to be approved in the USA, and Europe for the treatment of HIV infection. Zalcitabine was marketed by Roche under the trade name of Hivids. On December 31, 2006 Roche withdrew zalcitabine from sale in the USA, presumably because of the fact that newer antiretroviral drugs, especially abacavir, stavudine, and tenofovir, were more effective, less toxic, and had more favorable dosing schedules than zalcitabine. To the authors’ knowledge, zalcitabine is no longer used for the treatment of HIV infection anywhere in the world.
MECHANISM OF DRUG ACTION
Zalcitabine is a pyrimidine analog of the nucleoside deoxycytidine. Like other nucleoside analogs, the antiretroviral activity of zalcitabine is dependent upon the intracellular phosphorylation of zalcitabine to the triphosphate. Zalcitabine triphosphate reversibly inhibits the HIV reverse transcriptase by competing with the natural substrate (deoxycytidine). When incorporated into the growing HIV complementary DNA chain, it irreversibly inhibits further DNA synthesis by causing chain termination because it lacks the 3u hydroxyl group required for addition of further nucleotides. Zalcitabine enters the cell by both facilitated diffusion via the nucleoside carrier as well as by nonfacilitated diffusion. After entry into the cell, zalcitabine is sequentially phosphorylated to the mono-, di-, and triphosphate derivatives. The half-life of intracellular zalcitabine triphosphate in cells ranges from 2.6 to 10 hours, substantially longer than the plasma half-life of the unphosphorylated drug. There is no significant difference in the ability of HIV-infected and -uninfected T-cell lines to phosphorylate zalcitabine, although the antiviral and cellular cytotoxic effects of zalcitabine are cell-type dependent.
Drug interactions
There are no significant interactions between zalcitabine and zidovudine, based on studies in monkeys, and in HIV-infected patients enrolled in clinical trials of combination therapy with these drugs. Zalcitabine pharmacokinetics are also not altered by lamivudine. Zalcitabine should be ceased in patients requiring drugs known potentially to cause pancreatitis, including intravenous pentamidine. In addition, the drug should be avoided in patients receiving therapy with drugs that have the potential to cause peripheral neuropathy, e.g. vincristine, stavudine, and didanosine. Amphotericin, foscarnet, and aminoglycosides should be used with caution in patients receiving zalcitabine, with frequent clinical and biochemical monitoring, especially of renal function, and with consideration of dose adjustments. As zalcitabine is excreted predominantly by renal mechanisms, drugs that inhibit renal tubular function, such as probenecid, may increase serum levels of zalcitabine. There were no clinically significant pharmacokinetic interactions between ganciclovir and zalcitabine.
- Related articles
- Related Qustion
Didanosine (2u,3u-dideoxyinosine, ddI) is a synthetic purine nucleoside analog that is active against HIV-1 and HIV-2, including strains of HIV that are resistant to zidovudine and lamivudine.....
Apr 14,2022APICellulose acetate is used as a film base in photography, as a component in some adhesives, and as a frame material for eyeglasses; it is also used as a synthetic fiber and in the manufacture of cigarette filters.....
Apr 14,2022Catalyst and AuxiliaryZalcitabine
7481-89-2You may like
- Zalcitabine
-
- $0.00 / 1kg
- 2025-12-13
- CAS:7481-89-2
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Customise
- Zalcitabine
-
- $30.00 / 25mg
- 2025-12-10
- CAS:7481-89-2
- Min. Order:
- Purity: 99.08%
- Supply Ability: 10g
- Zalcitabine
-
- $30.00 / 25mg
- 2025-12-10
- CAS:7481-89-2
- Min. Order:
- Purity: 99.08%
- Supply Ability: 10g