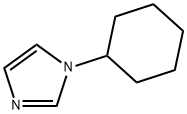
1-cyclohexyl-iMidazole synthesis
- Product Name:1-cyclohexyl-iMidazole
- CAS Number:67768-61-0
- Molecular formula:C9H14N2
- Molecular Weight:150.22

50-00-0

131543-46-9
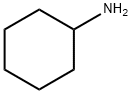
108-91-8

67768-61-0
The compound was synthesized by improving the method reported in the literature [22]. The procedure was as follows: in a 1L round bottom flask, ammonium chloride (10.7 g, 0.200 mol), glyoxal (40% aqueous solution, 15.3 mL, 0.134 mol), formaldehyde (30% aqueous solution, 16.0 mL, 0.134 mol) and deionized water (250 mL) were mixed well. Another flask was taken and cyclohexylamine (11.5 mL, 0.100 mol) was dissolved in deionized water (100 mL) and acidified with phosphoric acid (85%, 15 mL). The acidified cyclohexylamine solution was slowly added to the ammonium chloride mixture. The resulting colorless solution was heated and refluxed for 12 h. When the reaction was complete, it was cooled to room temperature to give a clarified orange solution. This solution was poured into a half-full of 1 L of ice and the pH was adjusted to 10-12 with 40% sodium hydroxide solution.Subsequently, the product was extracted with ethyl acetate and the organic layer was washed with water and dried with anhydrous sodium sulfate. After removing the solvent under reduced pressure, the product was purified by vacuum distillation (105 °C) to give 7.5 g of 1-cyclohexyl-1H-imidazole as a colorless oil in 50% yield. The structure of the product was confirmed by 1H NMR, 13C NMR and HR-EI-MS.1H NMR (400 MHz, CDCl3, 25°C) δ/ppm: 7.53 (s, 1H), 7.04 (s, 1H), 6.95 (s, 1H), 3.90 (tt, J = 11.8, 3.8 Hz, 1H), 2.12-2.09 (m, 2H), 1.92-1.92 (m, 1H), 2.12-2.09 (m, 1H), 2.12-2.09 (m, 1H), 2.12-2.09 (m, 1H), 2.12-2.09 (m, 1H). 2H), 1.92-1.87 (m, 2H), 1.79-1.70 (m, 1H), 1.67-1.57 (m, 2H), 1.45-1.35 (m, 2H), 1.29-1.18 (m, 1H). 13C NMR (101 MHz, CDCl3, 25°C) δ/ppm: 135.43, 129.03, 117.10, 56.90 117.10, 56.90, 34.54, 25.54, 25.34. HR-EI-MS [M]+ m/z: C9H14N2 calculated value 150.1157; measured value 150.1162.

50-00-0
896 suppliers
$10.00/25g

131543-46-9
1 suppliers
inquiry

108-91-8
630 suppliers
$10.00/5mL

67768-61-0
33 suppliers
$32.00/100mg
Yield:67768-61-0 50%
Reaction Conditions:
with phosphoric acid;ammonium chloride in water; for 12 h;Reflux;
Steps:
3.1 1-Cyclohexyl-1H-imidazole
This compound was prepared by amending a previously published method [22]. Ammonium chloride (10.7g, 0.200mol), glyoxal (40%, 15.3mL, 0.134mol), formaldehyde (30%, 16.0mL, 0.134mol), and deionized water (250mL) were combined in a 1L round bottom flask. In a separate flask, a solution of cyclohexylamine (11.5mL, 0.100mol) in deionized water (100mL) was acidified with phosphoric acid (85%, 15mL). The acidified amine was added to the ammonium chloride solution. After heating the resulting colorless solution at reflux for 12h, the clear orange solution was cooled to room temperature and subsequently poured into a 1L beaker that was half-filled with ice. The resulting mixture was made basic with sodium hydroxide (40%) until the pH was in the range of 10-12. The product was then extracted into ethyl acetate and the organic layer was washed with water and dried over sodium sulfate. Following removal of the solvent under reduced pressure, the desired product was purified via vacuum distillation (105°C) to give 7.5g of the title compound as a colorless oil. Yield=50%. 1H NMR (400MHz, CDCl3, 25°C) δ/ppm: 7.53 (s, 1H), 7.04 (s, 1H), 6.95 (s, 1H), 3.90 (tt, J=11.8, 3.8Hz, 1H), 2.12-2.09 (m, 2H), 1.92-1.87 (m, 2H), 1.79-1.70 (m, 1H), 1.67-1.57 (m, 2H), 1.45-1.35 (m, 2H), 1.29-1.18 (m, 1H). 13C NMR (101 MHZ, CDCl3, 25°C) δ/ppm: 135.43, 129.03, 117.10, 56.90, 34.54, 25.54, 25.34. HR-EI-MS [M]+ m/z: calculated for C9H14N2 150.1157; found, 150.1162.
References:
Andrade, Gabriel A.;DiMeglio, John L.;Guardino, Eric T.;Yap, Glenn P.A.;Rosenthal, Joel [Polyhedron,2017,vol. 135,p. 134 - 143]

288-32-4
1052 suppliers
$5.00/25g
110-83-8
321 suppliers
$11.00/25g

67768-61-0
33 suppliers
$32.00/100mg