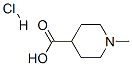
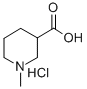
1-Methylpiperidine-4-carboxylic acid hydrochloride synthesis
- Product Name:1-Methylpiperidine-4-carboxylic acid hydrochloride
- CAS Number:71985-80-3
- Molecular formula:C7H14ClNO2
- Molecular Weight:179.64
Yield:71985-80-3 91%
Reaction Conditions:
Stage #1:isonipecotic acid;formaldehyd with formic acid;palladium 10% on activated carbon in water at 90 - 100;
Stage #2: with hydrogenchloride in water at 65 - 75;
Steps:
2.1
Charge isonipecotic acid (1.00 wt, l .Oeq, 600 g) to a reaction vessel. Charge palladium on charcoal (10%wt, 50% wet paste, 0.05wt, 30 g) to the reaction vessel. Charge purified water (4.0 vol, 2.4 L) to the reaction vessel. Heat the resulting mixture to 90 to 95°C. Charge formic acid (1.2 vol, 1.4wt, 4.0eq, 720 mL) to the vessel at 90 to 95°C(expected addition time 20 to 40 minutes). Charge a line rinse of purified water (0.5vol, 300 mL) to the vessel at 90 to 95°C. Charge formaldehyde (37% w/w aqueous solution, 0.74vol, 0.8 lwt, 1.3eq, 444 mL) to the vessel at 90 to 95°C (expected addition time 20 to 40 minutes). Charge a line rinse of purified water (0.5 vol, 300 mL) to the vessel at 90 to 95°C. Stir the resulting mixture at 90 to 100°C until complete by HPLC analysis (pass criterion <0.1% area isonipecotic acid, expected 3 hours). Cool the resulting mixture to 20 to 30°C. Filter the reaction mixture through GF/F. Wash the filter cake with purified water (2 x l.Ovol) at 20 to 30°C. Concentrate the combined filtrates to ca 2 vol at atmospheric pressure. As necessary adjust the temperature to 65 to 75°C. Charge cone. Hydrochloric acid (0.95 vol, 1.14wt, 1.5eq, 570 mL) to the vessel at 65 to 75°C. Charge acetonitrile (10.0 vol, 7.8 wt, 6.0 L) to the vessel at >70°C and concentrate the solution to ca 2 vol at atmospheric pressure. Charge acetonitrile (10.0 vol, 7.8 wt, 6.0 L) to the vessel at >70°C and concentrate the solution to ca 2 vol at atmospheric pressure. Charge acetonitrile (10.0 vol, 7.8wt, 6.0 L) to the vessel at >70oC and concentrate the solution to ca 3 vol at atmospheric pressure. Check the water content by KF analysis of the supernatant liquors using AX reagent (pass criterion<0.1%w/w). Cool the reaction mixture to 20 to 25°C. Stir the reaction mixture for 1 to 2 hours at 20 to 25°C. Filter the reaction mixture at 20 to 25°C. Wash the filter cake with acetonitrile (2 x 1.0 vol, 0.8 wt, 2 x 600 mL). Dry the product at up to 50°C until <0.5% w/w by LOD and <0.2%w/w water (KF, AX reagent). Expected yield: 80 to 90%th, 111 to 125%w/w; Isolated yield: 755 g (91%th, 125w/w). Figure 5 shows a typical NMR spectrum of l-Methylpiperidine-4-carboxylic acid (D20)
References:
COLUCID PHARMACEUTICALS, INC.;CARNIAUX, Jean-Francois;CUMMINS, Jonathan WO2011/123654, 2011, A1 Location in patent:Page/Page column 29-30
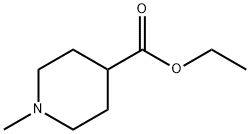
24252-37-7
173 suppliers
$6.00/1g

71985-80-3
153 suppliers
$6.00/1g
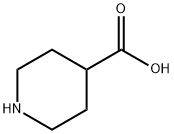
498-94-2
0 suppliers
$5.00/10g

71985-80-3
153 suppliers
$6.00/1g
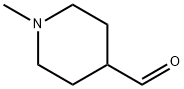
50675-21-3
79 suppliers
$45.00/50mg

24252-37-7
173 suppliers
$6.00/1g

71985-80-3
153 suppliers
$6.00/1g
19999-64-5
88 suppliers
$10.00/1g
