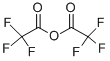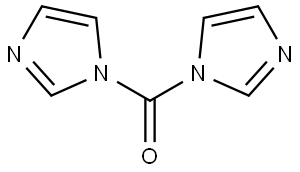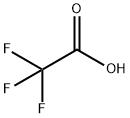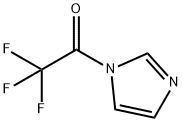
1-(Trifluoroacetyl)imidazole synthesis
- Product Name:1-(Trifluoroacetyl)imidazole
- CAS Number:1546-79-8
- Molecular formula:C5H3F3N2O
- Molecular Weight:164.09
Yield: 79%
Reaction Conditions:
in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
4.3. N-(trifluoroacetyl)imidazole
This compound was prepared by modified procedure publishedearlier.14 To a pre-cooled (5 C) and well-stirred absolute THF(100 mL) trifluoroacetic anhydride (80 g, 380 mmol) was addedcarefully. The solution formed was added to a pre-cooled (5 C) andwell-stirred solution of imidazole (51.6 g, 759 mmol) in absoluteTHF (240 mL) carefully. The mixture was stirred for 1 h at ambienttemperature, and left at 0 C overnight. The solid precipitate wasfiltered off and washed with diethyl ether (25 mL). The filtrate wasconcentrated in vacuo without heating and the residuewas distilledto provide the desired product. Yield 49 g (79%), hydrolyticallysensitive colorless liquid, very moisture sensitive, bp 73e75 C(100 Torr) (lit.14 45e46 C (14 Torr)). 1H NMR (CDCl3) d: 7.21 (dd, 1H,H-4, J 1.8, 0.8 Hz), 7.55 (m, 1H, H-5), 8.24 (s, 1H, H-2). 19F NMR(CDCl3) d: 71.3 (s, CF3). The parameters in agreement with published data.15
References:
Sevenard, Dmitri V.;Didenko, Andrey V.;Lorenz, Denis;Vorobiev, Michael;Stelten, Johannes;Dülcks, Thomas;Sosnovskikh, Vyacheslav Ya. [Tetrahedron,2017,vol. 73,# 11,p. 1495 - 1502]
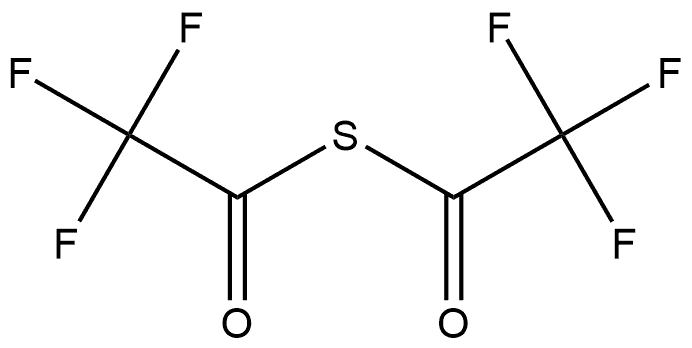
130451-27-3
0 suppliers
inquiry
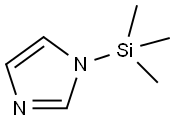
18156-74-6
449 suppliers
$8.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1546-79-8
152 suppliers
$12.50/5G