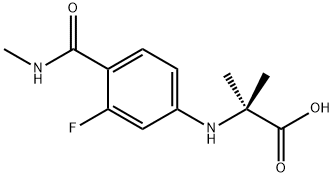
N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine synthesis
- Product Name:N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine
- CAS Number:1289942-66-0
- Molecular formula:C12H15FN2O3
- Molecular Weight:254.26
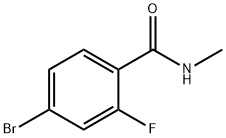
749927-69-3
62-57-7
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine](/CAS/20150408/GIF/1289942-66-0.gif)
1289942-66-0
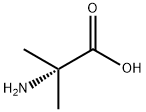
The general procedure for the synthesis of 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropanoic acid from 4-bromo-2-fluoro-N-methylbenzamide and 2-aminoisobutyric acid was as follows: 4-bromo-2-fluoro-N-methylbenzamide (10 g, 43.1 mmol), 2-aminoisobutyric acid (6.7 g, 64.7 mmol), potassium carbonate ( 23.8 g, 172.4 mmol) and proline (0.7 g, 4.31 mmol) were added sequentially to a single-neck flask. Subsequently, water (1.8 ml, 100 mmol) was dissolved in DMF (60 ml) and this solution was added to the flask. Under nitrogen protection, CuCl (0.45 g, 4.31 mmol) was added to the reaction mixture, and then the reaction system was heated to 100 °C and the reaction was continuously stirred for 24 hours. After completion of the reaction, the reaction mixture was diluted with water and extracted with dichloromethane. After separation of the organic phase, the pH of the aqueous phase was adjusted to 4 with 1 mol/L citric acid solution, at which point a solid precipitated. The solid was collected by filtration and washed three times with a mixture of water and ethanol (100:1, v/v), resulting in 9.5 g of pure white solid product in 87% yield.

749927-69-3
308 suppliers
$40.00/1g

62-57-7
461 suppliers
$10.64/25G
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine](/CAS/20150408/GIF/1289942-66-0.gif)
1289942-66-0
232 suppliers
$11.00/1g
Yield:1289942-66-0 88.7%
Reaction Conditions:
with 2-acetylcyclohexanone;potassium carbonate;copper(l) chloride in N,N-dimethyl-formamide at 110; for 16 h;
Steps:
1 Example 1: Synthesis of 2- (3-fluoro-4- (methylcarbamoyl) phenylamino) -2-methylpropanoic acid
N-methyl-4-bromo-2-fluoro-benzamide (A) (600 g, 2.6 mol, 1.0 eq), 2-amino-2-methylpropionic acid (400 g, 3.9 mol, 1.5 eq) Potassium carbonate (893g, 6.5mol, 2.5eq), CuCl (51g, 0.52mol, 0.2eq), add to DMF (3600ml), stir well, add 2-acetylcyclohexanone (73g, 0.52mol, 0.2eq) ), Reaction at 110 ° C for 16 hours under inert gas. After the reaction was completed, the reaction solution was cooled to room temperature, purified water and ethyl acetate were added and extracted twice, and the organic layers were combined. The organic layer was adjusted to be acidic with a 1N hydrochloric acid solution, crystallized by stirring at 0-5 ° C, filtered, the filter cake was washed with purified water, and dried at 60 ° C to obtain 583 g of a yellow solid with a yield of 88.7%.
References:
CN110872258,2020,A Location in patent:Paragraph 0015

749927-69-3
308 suppliers
$40.00/1g
15028-41-8
220 suppliers
$9.00/10g
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine](/CAS/20150408/GIF/1289942-66-0.gif)
1289942-66-0
232 suppliers
$11.00/1g

292621-45-5
94 suppliers
$35.00/250mg
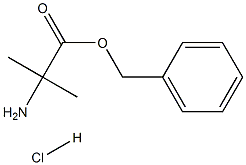
60421-20-7
68 suppliers
$79.00/1g
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine](/CAS/20150408/GIF/1289942-66-0.gif)
1289942-66-0
232 suppliers
$11.00/1g
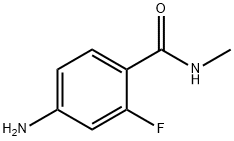
915087-25-1
236 suppliers
$8.00/250mg
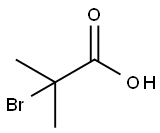
2052-01-9
272 suppliers
$10.00/1g
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine](/CAS/20150408/GIF/1289942-66-0.gif)
1289942-66-0
232 suppliers
$11.00/1g
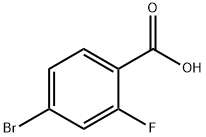
112704-79-7
534 suppliers
$15.00/5G
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine](/CAS/20150408/GIF/1289942-66-0.gif)
1289942-66-0
232 suppliers
$11.00/1g