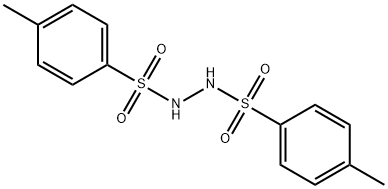
1,2-Bis(p-tolylsulfonyl)hydrazine, N,Nμ-Ditosylhydrazine, 4-Methylbenzenesulfonic acid 2-[(4-methylphenyl)sulfonyl]hydrazide synthesis
- Product Name:1,2-Bis(p-tolylsulfonyl)hydrazine, N,Nμ-Ditosylhydrazine, 4-Methylbenzenesulfonic acid 2-[(4-methylphenyl)sulfonyl]hydrazide
- CAS Number:14062-05-6
- Molecular formula:C14H16N2O4S2
- Molecular Weight:340.42

98-59-9
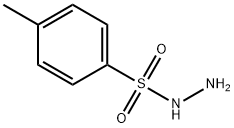
1576-35-8
![1,2-Bis(p-tolylsulfonyl)hydrazine, N,Nμ-Ditosylhydrazine, 4-Methylbenzenesulfonic acid 2-[(4-methylphenyl)sulfonyl]hydrazide](/CAS/GIF/14062-05-6.gif)
14062-05-6
Example 6 - Preparation of N,N'-bis(p-toluenesulfonyl)hydrazine: To a solution of dichloromethane (50 mL) containing p-toluenesulfonylhydrazine (9.32 g, 50 mmol) and p-toluenesulfonyl chloride (14.3 g, 75 mmol) was added pyridine (6 mL, 75 mmol). The reaction mixture was stirred at room temperature for 1.5 hours. Subsequently, ether (200 mL) and water (100 mL) were added and stirring was continued at 0°C for 15 minutes. After completion of the reaction, the mixture was filtered and the solid product was washed with ether. Finally, purification by recrystallization from methanol afforded N,N'-bis(p-toluenesulfonyl)hydrazine as a white solid (11.5 g, 68% yield).
104-15-4
764 suppliers
$133.00/500g
![1,2-Bis(p-tolylsulfonyl)hydrazine, N,Nμ-Ditosylhydrazine, 4-Methylbenzenesulfonic acid 2-[(4-methylphenyl)sulfonyl]hydrazide](/CAS/GIF/14062-05-6.gif)
14062-05-6
64 suppliers
$11.00/250mg
Yield: 95%
Reaction Conditions:
with carbazic acid in neat (no solvent) at 100; for 5 h;Green chemistry;
Steps:
18
General procedure: 7.6 g (100.0 mmol) of solid hydrazine (H3N + NHCO2-) and adipic acid (7.3 g, 50.0 mmol) were mixed in a mortar without a solvent for 10 minutes and stirred at 100 ° C for 5 hours. To confirm the structure and composition of the product produced in this process and analyzed using 400 MHz NMR (nuclear magnetic resonance) and elemental analysis) .As a result of the analysis, the product was adipohydrazide (C6H14N4O2), the conversion rate was 96% or more, and the yield was 95% or more. Yield (8.27 g, 96% or more); The product was obtained in the same manner as in Example 1 except that 8.61 g of 4-methylbenzenesulfonic acid was used instead of adipic acid. As a result of the analysis, the product was 4-methyl-N'-tosylbenzenesulfonohydrazide, the selectivity was 97%, and the yield was 95%. Yield: (8.08g, 95%).
References:
Sogang University Academic Cooperation;Haw, Nam Hwe;Lee, Byung Noh KR2015/88523, 2015, A Location in patent:Paragraph 0128; 0218-0220

98-59-9
626 suppliers
$9.00/5g

1576-35-8
468 suppliers
$10.00/1g
![1,2-Bis(p-tolylsulfonyl)hydrazine, N,Nμ-Ditosylhydrazine, 4-Methylbenzenesulfonic acid 2-[(4-methylphenyl)sulfonyl]hydrazide](/CAS/GIF/14062-05-6.gif)
14062-05-6
64 suppliers
$11.00/250mg

98-59-9
626 suppliers
$9.00/5g
![1,2-Bis(p-tolylsulfonyl)hydrazine, N,Nμ-Ditosylhydrazine, 4-Methylbenzenesulfonic acid 2-[(4-methylphenyl)sulfonyl]hydrazide](/CAS/GIF/14062-05-6.gif)
14062-05-6
64 suppliers
$11.00/250mg
120-46-7
426 suppliers
$6.00/25g

1576-35-8
468 suppliers
$10.00/1g
![1,2-Bis(p-tolylsulfonyl)hydrazine, N,Nμ-Ditosylhydrazine, 4-Methylbenzenesulfonic acid 2-[(4-methylphenyl)sulfonyl]hydrazide](/CAS/GIF/14062-05-6.gif)
14062-05-6
64 suppliers
$11.00/250mg

1576-35-8
468 suppliers
$10.00/1g
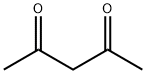
123-54-6
588 suppliers
$10.00/25ml
![1,2-Bis(p-tolylsulfonyl)hydrazine, N,Nμ-Ditosylhydrazine, 4-Methylbenzenesulfonic acid 2-[(4-methylphenyl)sulfonyl]hydrazide](/CAS/GIF/14062-05-6.gif)
14062-05-6
64 suppliers
$11.00/250mg