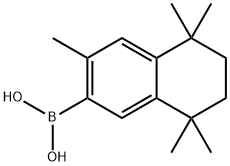
3,5,5,8,8-PENTAMETHYL-5,6,7,8-TETRAHYDRONAPHTHALEN-2-YLBORONIC ACID synthesis
- Product Name:3,5,5,8,8-PENTAMETHYL-5,6,7,8-TETRAHYDRONAPHTHALEN-2-YLBORONIC ACID
- CAS Number:169126-64-1
- Molecular formula:C15H23BO2
- Molecular Weight:246.15
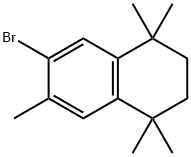
119999-22-3

169126-64-1
Synthesis was carried out using the method of Faul et al. THF (30 mL) was added to a 100 mL round-bottomed flask, followed by the addition of a hexane solution of 1.6 M n-butyllithium (8.0 mL, 12.8 mmol). The resulting solution was cooled to -78°C in a dry ice-acetone bath and stirred under nitrogen protection. a solution of a 2:1 mixture of 6-bromo-1,1,4,4,7-pentamethyl-1,2,3,4-tetrahydronaphthalene (3.3587 g, 7.88 mmol) in THF (8 mL) was added dropwise to this solution over a 20-min period, and the reaction mixture continued to stir at -78°C for for 10 min. Subsequently, a solution of triisopropyl borate (4.9 mL, 21.3 mmol) in THF (10 mL) was added dropwise over 20 minutes. The reaction was stirred at -78°C for 1 h. After stirring, the reaction was slowly warmed to room temperature and stirring was continued for 2 h. The reaction was then purged with 3N HCl (2.5 mL). Upon completion of the reaction, the reaction was quenched with 3N HCl (35 mL) and stirred for 2 h. After stirring, the mixture was poured into ethyl acetate and the organic and aqueous layers were separated. The aqueous layer was extracted with ethyl acetate and the combined organic layers were washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuum to give the crude product. The crude product was purified by column chromatography (150 mL silica gel, ethyl acetate:hexane=1:3) to afford 3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaphthalene-2-boronic acid (0.8838 g, 45%) as a white crystalline solid. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3) and 13C NMR (100.6 MHz, CDCl3).

5419-55-6
388 suppliers
$12.00/5g

119999-22-3
88 suppliers
$22.00/1g

169126-64-1
66 suppliers
$73.00/250mg
Yield:169126-64-1 73%
Reaction Conditions:
Stage #1: 2-bromo-3-methyl-5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalenewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.333333 h;Inert atmosphere;
Stage #2: Triisopropyl borate in tetrahydrofuran;hexane at -78; for 2 h;Inert atmosphere;
Stage #3: with hydrogenchloride;water in tetrahydrofuran;hexane at 20; for 1 h;
References:
Yamada, Shoya;Kawasaki, Mayu;Fujihara, Michiko;Watanabe, Masaki;Takamura, Yuta;Takioku, Maho;Nishioka, Hiromi;Takeuchi, Yasuo;Makishima, Makoto;Motoyama, Tomoharu;Ito, Sohei;Tokiwa, Hiroaki;Nakano, Shogo;Kakuta, Hiroki [Journal of Medicinal Chemistry,2019,vol. 62,# 19,p. 8809 - 8818]

119999-22-3
88 suppliers
$22.00/1g

169126-64-1
66 suppliers
$73.00/250mg

13780-71-7
3 suppliers
inquiry

121-43-7
382 suppliers
$15.00/25ml

119999-22-3
88 suppliers
$22.00/1g

169126-64-1
66 suppliers
$73.00/250mg
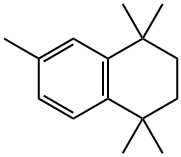
6683-48-3
150 suppliers
$8.00/1g

169126-64-1
66 suppliers
$73.00/250mg
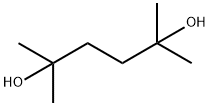
110-03-2
360 suppliers
$6.00/25g

169126-64-1
66 suppliers
$73.00/250mg