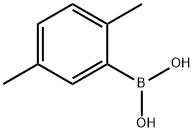
2,5-Dimethylphenylboronic acid synthesis
- Product Name:2,5-Dimethylphenylboronic acid
- CAS Number:85199-06-0
- Molecular formula:C8H11BO2
- Molecular Weight:149.98

121-43-7
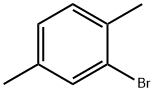
553-94-6

7732-18-5

85199-06-0
The general procedure for the synthesis of 2,5-dimethylphenylboronic acid from trimethyl borate, 2,5-dimethylbromobenzene and aqueous electrophoretic grade was as follows: 1-bromo-2,5-dimethylbenzene (25.0 g, 0.135 mol) was dissolved in 200 ml of tetrahydrofuran under the protection of nitrogen in a 1 L round-bottomed flask and cooled to -78 °C. A 1.6 M n-butyllithium solution was added slowly dropwise and the reaction was stirred for 2 hours keeping the temperature at -78 °C. Subsequently, trimethyl borate (18.2 g, 0.176 mol) was added dropwise and the reaction continued at the same temperature. After completion of the reaction, 1 equivalent of hydrochloric acid (101 ml, 0.162 mol) was added to acidify the reaction mixture at room temperature and stirred for 1 hour. The reaction solution was concentrated under reduced pressure and the resulting solid was recrystallized from hexane to give Intermediate 1-b (12.5 g, 62% yield).

121-43-7
382 suppliers
$13.00/25mL

553-94-6
204 suppliers
$10.00/5g

7732-18-5
507 suppliers
$12.69/100ml

85199-06-0
262 suppliers
$6.00/1g
Yield: 62%
Reaction Conditions:
Stage #1:4-bromo-m-xylene with n-butyllithium in tetrahydrofuran at -78; for 2 h;Inert atmosphere;
Stage #2:Trimethyl borate in tetrahydrofuran at -78;Inert atmosphere;
Stage #3:water with hydrogenchloride in tetrahydrofuran at 20; for 1 h;Inert atmosphere;
Steps:
1-(2) Synthesis of [Intermediate 1-b]
1L round bottom flask, 1-bromo-2,5-dimethylbenzene (25.0g, 0.135mol), into 200mlof tetrahydrofuran was cooled to -78 ° C under a nitrogen atmosphere.To the cooled solution was added dropwise a 1.6M n-butyllithium (101ml, 0.162mol).After 2 hours stirring at the same temperature was added dropwise trimethyl borate(18.2g, 0.176mol).Put the 1 normal hydrochloric acid and then stirred for 1 hour at room temperature thesolution was acidified.The mixture was concentrated under reduced pressure, and recrystallized with nhexaneto give the [intermediate 1-b].(12.5g, 62%)
References:
SFC Co., Ltd.;Cha, Sun Wook;Jung, Kyung Suk;Park, Suk Bae;Kim, Hee Dae;Lee, Yu Lim;Song, Joo Man;Hwang, Moon Chan KR2015/52989, 2015, A Location in patent:Paragraph 0326-0331

553-94-6
204 suppliers
$10.00/5g
11113-50-1
297 suppliers
$34.40/100 g

85199-06-0
262 suppliers
$6.00/1g
30897-86-0
57 suppliers
$130.00/10g

85199-06-0
262 suppliers
$6.00/1g

121-43-7
382 suppliers
$13.00/25mL

553-94-6
204 suppliers
$10.00/5g

85199-06-0
262 suppliers
$6.00/1g

553-94-6
204 suppliers
$10.00/5g

85199-06-0
262 suppliers
$6.00/1g