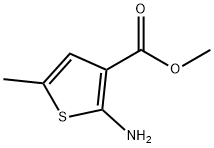
2-AMINO-5-METHYL-THIOPHENE-3-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:2-AMINO-5-METHYL-THIOPHENE-3-CARBOXYLIC ACID METHYL ESTER
- CAS Number:19369-53-0
- Molecular formula:C7H9NO2S
- Molecular Weight:171.22
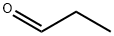
123-38-6
105-34-0

19369-53-0
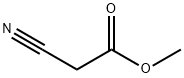
1. Sulfur powder (960.0 mg, 30.0 mmol) and 5 mL of DMF were added to a single-necked vial and stirred until dissolved. 2. Methyl cyanoacetate (3.39 g, 30.0 mmol) and morpholine (1.48 g, 17.0 mmol) were added to the reaction vial sequentially, and the reaction solution gradually turned dark brown. 3. Propionaldehyde (1.74 g, 30.0 mmol) was added and the reaction mixture was stirred at 50 °C overnight. 4. After completion of the reaction, the mixture was cooled to room temperature and diluted with an appropriate amount of water. 5. The reaction mixture was extracted with ethyl acetate three times and the organic phases were combined. 6. 6. The combined organic phases were washed with water and then saturated brine. 7. 7. the organic phase was dried with anhydrous magnesium sulfate, filtered and distilled under reduced pressure to remove the solvent, the crude product was obtained. 8. The crude product was separated and purified by column chromatography to obtain the yellowish solid product methyl 2-amino-5-methylthiophene-3-carboxylate. Yield: 73.6%.
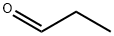
123-38-6
455 suppliers
$14.00/5mL

105-34-0
458 suppliers
$5.00/25g

19369-53-0
99 suppliers
$10.00/500mg
Yield: 73.6%
Reaction Conditions:
with morpholine;sulfur in N,N-dimethyl-formamide at 50;
Steps:
27.a a) 2-amino-5-methylthiophene-3-carboxylic acid methyl ester (27a)
The sulfur powder (960.0mg, 30.0mmol)In a single mouth bottle,Add DMF 5 mL,Were successively added methyl cyanoacetate (3.39g, 30.0mmol),Morpholine (1.48 g, 17.0 mmol),The reaction solution was dark brown,Propionaldehyde (1.74 g, 30.0 mmol) was added,50 ° C overnight.After completion of the reaction, the mixture was cooled to room temperature,Add appropriate amount of water,Ethyl acetate was extracted three times,The combined organic phases were washed with saturated brine,Dried over anhydrous magnesium sulfate,The crude product was distilled under reduced pressure.Separation and purification by column chromatography,To give a pale yellow solid.Yield: 73.6%
References:
Zhejiang University;Shanghai Institute of Materia Medica, Chinese Academy of Sciences;Chen, Jianzhong;Xie, Xin;Qian, Haiyan;Chen, Lili;Wang, Zhilong CN106167497, 2016, A Location in patent:Paragraph 0241; 0242; 0243