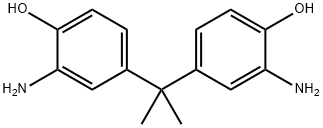
2,2-Bis(3-amino-4-hydroxyphenyl)propane synthesis
- Product Name:2,2-Bis(3-amino-4-hydroxyphenyl)propane
- CAS Number:1220-78-6
- Molecular formula:C15H18N2O2
- Molecular Weight:258.32

Thedinitro compound (2) (10,000 g, 0.0314 mol) was heated to reflux inabsolute ethanol (100 mL) in the presence of wet 10% Pd/C catalyst(0.650 g). Hydrazine monohydrate (20 mL) was then added dropwiseover a period of 30 min, and the reaction was maintained at reflux for24 h. The hot solution was filtered through Celite to eliminate thecatalyst and partially concentrated under reduced pressure. Afterwards,the concentrate was poured into cold distilled water. The crude productwas filtered, washed with water, dried under vacuum and recrystallizedtwice from methanol to give (3). The diamino compound was obtainedas a white powder: yield: 88%; mp. 263 °C (DSC); Analysis: Calculatedfor C15H18N2O2: C, 69.74%; H, 7.02%; N, 10.84%. Found: C, 69.35%;H, 7.15%; N, 11.02%. 1H NMR (400 MHz, DMSO-d6): δ (ppm); 8.57 (s,1H, OH), 6.43 (d, J=8.1 Hz, 1H, Har), 6.34 (d, J=1.8 Hz, 1H, Har),6.21 (dd, J=8.1 Hz, 1.8 Hz, 1H, Har), 4.23 (s, 2H, NH) 1.37 (s, 3H, CH3f). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 142.4, 141.4, 135.4, 114.4,113.5, 113.4, 40.8, 30.9.
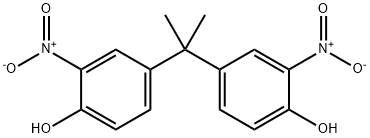
5329-21-5
5 suppliers
inquiry

1220-78-6
156 suppliers
$6.00/1g
Yield:1220-78-6 88%
Reaction Conditions:
with palladium 10% on activated carbon;hydrazine hydrate in ethanol; for 24 h;Reflux;
Steps:
Synthesis of 2,2-bis(3-amino-4-hydroxyphenyl) propane (3):
Thedinitro compound (2) (10,000 g, 0.0314 mol) was heated to reflux inabsolute ethanol (100 mL) in the presence of wet 10% Pd/C catalyst(0.650 g). Hydrazine monohydrate (20 mL) was then added dropwiseover a period of 30 min, and the reaction was maintained at reflux for24 h. The hot solution was filtered through Celite to eliminate thecatalyst and partially concentrated under reduced pressure. Afterwards,the concentrate was poured into cold distilled water. The crude productwas filtered, washed with water, dried under vacuum and recrystallizedtwice from methanol to give (3). The diamino compound was obtainedas a white powder: yield: 88%; mp. 263 °C (DSC); Analysis: Calculatedfor C15H18N2O2: C, 69.74%; H, 7.02%; N, 10.84%. Found: C, 69.35%;H, 7.15%; N, 11.02%. 1H NMR (400 MHz, DMSO-d6): δ (ppm); 8.57 (s,1H, OH), 6.43 (d, J=8.1 Hz, 1H, Har), 6.34 (d, J=1.8 Hz, 1H, Har),6.21 (dd, J=8.1 Hz, 1.8 Hz, 1H, Har), 4.23 (s, 2H, NH) 1.37 (s, 3H, CH3f). 13C NMR (100 MHz, DMSO-d6): δ (ppm) 142.4, 141.4, 135.4, 114.4,113.5, 113.4, 40.8, 30.9.
References:
Díez, Blanca;Cuadrado, Purificación;Marcos-Fernández, ángel;de la Campa, José G.;Tena, Alberto;Prádanos, Pedro;Palacio, Laura;Lee, Young Moo;Alvarez, Cristina;Lozano, ángel E.;Hernández, Antonio [Reactive and functional polymers,2018,vol. 127,p. 38 - 47]
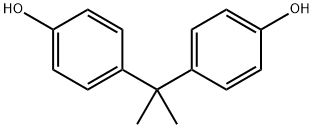
80-05-7
709 suppliers
$13.00/100g

1220-78-6
156 suppliers
$6.00/1g