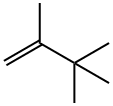
2,3,3-TRIMETHYL-1-BUTENE synthesis
- Product Name:2,3,3-TRIMETHYL-1-BUTENE
- CAS Number:594-56-9
- Molecular formula:C7H14
- Molecular Weight:98.19

67-56-1
790 suppliers
$7.29/5ml-f
78-78-4
281 suppliers
$20.00/25ML
79-29-8
138 suppliers
$20.00/1g
464-06-2
96 suppliers
$23.50/1g

594-56-9
58 suppliers
$39.00/1mL
87-85-4
181 suppliers
$25.19/1g
Yield:-
Reaction Conditions:
with hypophosphorous acid;isopropyl alcohol;zinc(II) iodide in water at 150 - 200; for 3 - 66 h;Product distribution / selectivity;
Steps:
17; 18; 19; 22; 24; 26; 30; 31; 32; 33; 34
Experiments Using Hypophosphorous Acid:[0059] Hypophosphorous acid, H3PO2 is commercial available as an aqueous solution (50%) and the most stable tautomer is H2P(O)OH.[0060] In a typical experiment, an aqueous solution of hypophosphorous acid was charged into a thick-wall pressure vessel and was subjected to a vacuum for 12-36 hours, followed by the addition of zinc iodide (ZnI2) (32 mol%), methanol (791 mg), and, if applicable, iso-propanol (/-PrOH). The reaction vessel was dipped into a preheated oil bath and was heated for a certain time, during which time white precipitates appeared. In most cases, the mixture was heated for 6-66 hours till the precipitates disappeared, depending on the loading of hypophosphorous acid and whether iso-propanol was used. In the cases where the amount of hypophosphorous acid was above 7.4 mol%, no dissolving of these precipitates had been observed and the reaction vessel was removed from the oil bath before the mixture turned light orange. The vessel was cooled to room temperature and the products were analyzed by GC or NMR.[0061] As a comparison between H3PO3 and H3PO2 (7.4 mol%) (Examples 17 and 18), the reaction at 200 0C showed an increase of yield (based on total carbon) from to 26% for phosphorous acid (H3PO3) to 33% for hypophosphorous acid (H3PO2). The most striking differences between the reaction mixtures is that the thptane to triptene ratio is over 20:1 for the reaction using hypophosphorous acid based on both GC and 13C NMR analysis. Figure 1 shows the 13C NMR spectrum of organic products obtained using the present methods with H3PO2 provided as an additive, and Figure 2 shows the 13C NMR spectrum of the organic products obtained without any phosphorus compound present. The 13C NMR spectrum (Figure 1 ) of the organic products with H3PO2 additive had a higher concentration of thptane than that without (Figure 2) although
References:
CALIFORNIA INSTITUTE OF TECHNOLOGY;BP CHEMICALS LIMITED WO2008/24896, 2008, A2 Location in patent:Page/Page column 15-18
75-97-8
245 suppliers
$10.00/1g

594-56-9
58 suppliers
$39.00/1mL
594-83-2
43 suppliers
inquiry

594-56-9
58 suppliers
$39.00/1mL
187737-37-7
1 suppliers
inquiry
115-10-6
108 suppliers
$75.00/25 g
106-98-9
169 suppliers
$10.00/25 g

464-06-2
96 suppliers
$23.50/1g

594-56-9
58 suppliers
$39.00/1mL
115-11-7
243 suppliers
$45.00/25 g