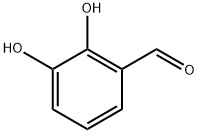
2,3-Dihydroxybenzaldehyde synthesis
- Product Name:2,3-Dihydroxybenzaldehyde
- CAS Number:24677-78-9
- Molecular formula:C7H6O3
- Molecular Weight:138.12
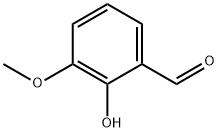
148-53-8

24677-78-9
The general procedure for the synthesis of 2,3-dihydroxybenzaldehyde from o-vanillin was as follows: acetonitrile (40 ml) was added to a 100 ml aubergine flask, followed by sequential addition of aluminum trichloride (0.752 g, 5.64 mmol, 1.1 equiv), sodium iodide (2.305 g, 15.38 mmol, 3.0 equiv) and o-vanillin (0.780 g, 5.13 mmol). The reaction mixture was heated to 80 °C and the reaction was stirred for 18 hours. After completion of the reaction, the heating was stopped and cooled to room temperature. The reaction solution was acidified with 2 mol/L dilute hydrochloric acid (10 ml) and subsequently extracted with ethyl acetate (50 ml x 3). The organic phases were combined and washed sequentially with saturated aqueous sodium thiosulfate (10 ml) and saturated brine (10 ml). The organic phase was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. Purification by column chromatography afforded 2,3-dihydroxybenzaldehyde (0.620 g, yellow solid, 87% yield).

148-53-8
446 suppliers
$10.00/1g

24677-78-9
268 suppliers
$8.00/1g
Yield:24677-78-9 87%
Reaction Conditions:
with aluminum (III) chloride;sodium iodide in acetonitrile at 80; for 18 h;
Steps:
2
Add acetonitrile (40ml) to a 100ml eggplant bottle,Aluminum trichloride (0.752g, 5.64mmol, 1.1eq),NaI (2.305 g, 15.38 mmol, 3.0 eq) and o-vanillin (0.780 g, 5.13 mmol),Heat to 80 ° C, stop stirring after 18 hours of reaction.After cooling to room temperature, it was acidified with 2 mol/L of dilute hydrochloric acid (10 ml), and extracted with ethyl acetate (50 ml × 3). The organic phase was combined and washed with saturated aqueous sodium thiosulfate (10 ml). Washed with saturated brine (10 ml), dried over anhydrous magnesium sulfate, filtered, evaporated, evaporated, evaporated. )purification,0.620 g of 2,3-dimethoxybenzaldehyde were obtained (yellow solid, yield 87%).
References:
Jingchu Institute Of Technology;Sang Dayong;Tu Xiaodong;Tian Juan CN108821955, 2018, A Location in patent:Paragraph 0021; 0022; 0025; 0026
67-66-3
0 suppliers
$33.60/500ml
120-80-9
533 suppliers
$13.00/25g

24677-78-9
268 suppliers
$8.00/1g

50-00-0
898 suppliers
$10.00/25g

120-80-9
533 suppliers
$13.00/25g

24677-78-9
268 suppliers
$8.00/1g
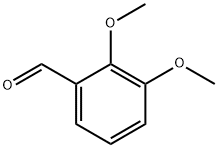
86-51-1
410 suppliers
$6.00/5g

24677-78-9
268 suppliers
$8.00/1g
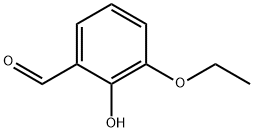
492-88-6
131 suppliers
$13.43/1gm:

24677-78-9
268 suppliers
$8.00/1g