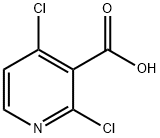
2,4-Dichloropyridine-3-carboxylic acid synthesis
- Product Name:2,4-Dichloropyridine-3-carboxylic acid
- CAS Number:262423-77-8
- Molecular formula:C6H3Cl2NO2
- Molecular Weight:192
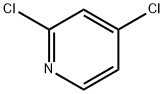
26452-80-2

124-38-9

262423-77-8
GENERAL STEPS: A THF solution (28.4 mL, 56.8 mmol) of 2M lithium diisopropylammonium (LDA) was slowly added to a tetrahydrofuran (THF, 70 mL) solution of 2,4-dichloropyridine (7 g, 47.3 mmol) at -78 °C, maintaining the temperature at -78 °C and stirring for 30 min. Upon completion of the reaction, the reaction mixture was quenched with excess dry ice, followed by warming to room temperature and continued stirring for 30 min. After neutralizing the reaction mixture with 1.5 N hydrochloric acid (HCl), it was diluted with ethyl acetate (100 mL) and washed sequentially with saturated brine (2 x 50 mL) and deionized water (100 mL). The organic layer was separated, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure to afford 2,4-dichloronicotinic acid (4.5 g, 23.44 mmol, 50% yield) as a brown solid. The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 8.47 (d, 1H), 7.74 (d, 1H).

124-38-9
134 suppliers
$175.00/23402

262423-77-8
206 suppliers
$9.00/1g
Yield:262423-77-8 82%
Reaction Conditions:
Stage #1:2,4-dichloropyridine with n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2:carbon dioxide in tetrahydrofuran;hexane at -78 - 20; for 0.166667 h;
Stage #3: with hydrogenchloride;water; pH=4
Steps:
Preparation of 2,4-Dichloronicotinic acid To a stirring solution of diisopropyl ethyl amine (11.1 ml, 81.08 mmol) in THF (50 ml) was added dropwise a solution of BuLi (1.46 M, 43.3 ml, 73.65 mmol) in hexane below -65° C. and the mixture was stirred for 40 minutes. To this solution was added dropwise 2,4-dichloropyridine (10 g, 67.57 mmol) in THF (15 mL) at -78° C. and stirred for 30 minutes. Carbon dioxide generated from freshly crushed dry ice was passed through CaCl2 guard tube and then charged into the reaction mixture for 10 minutes and the reaction mixture was slowly allowed to come to room temperature. The solvent was evaporated under reduced pressure and dissolved in a minimum volume of water. The aqueous layer was washed with water and acidified to pH 4 with conc. HCl. It was then extracted with ethyl acetate, the organic layer was washed with brine and dried over sodium sulfate. The organic solvent was removed under reduced pressure to provide 2,4-dichloronicotinic acid (10.6 g, 82%) as off white solid. 1H NMR (400 MHz, DMSO-d6): δ 14.88-14.54 (br s, 1H), 8.46 (d, J=5.3 Hz, 1H), 7.74 (d, J=5.5 Hz, 1H). FIA MS [M+H]: 191.8
References:
Pfizer Inc US2008/85887, 2008, A1 Location in patent:Page/Page column 17

26452-80-2
407 suppliers
$7.00/5g

124-38-9
134 suppliers
$175.00/23402

262423-77-8
206 suppliers
$9.00/1g

26452-80-2
407 suppliers
$7.00/5g

262423-77-8
206 suppliers
$9.00/1g
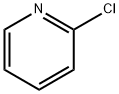
109-09-1
0 suppliers
$10.60/1gm:

262423-77-8
206 suppliers
$9.00/1g
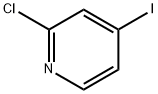
153034-86-7
384 suppliers
$10.00/1g

262423-77-8
206 suppliers
$9.00/1g