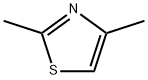
2,4-Dimethylthiazole synthesis
- Product Name:2,4-Dimethylthiazole
- CAS Number:541-58-2
- Molecular formula:C5H7NS
- Molecular Weight:113.18
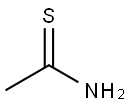
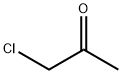
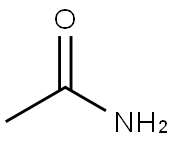
62-55-5
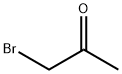
598-31-2

541-58-2
GENERAL METHOD: Thioacetamide (0.75 g, 10 mmol) and bromoacetone (1.99 g, 10 mmol) were dissolved in 5 mL of DMF in a 20 mL pressure tube. The reaction mixture was sealed and heated in an oil bath at 60 °C for 1 hour. After the reaction was completed, the reaction mixture was diluted with saturated aqueous ammonium chloride solution. Subsequently, the target product 2,4-dimethylthiazole was extracted with ethyl acetate. The combined organic layers were dried with anhydrous magnesium sulfate, filtered and concentrated. Finally, the crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate) to afford 2,4-dimethylthiazole in 99% yield as a white solid.
Yield:541-58-2 75%
Reaction Conditions:
in N,N-dimethyl-formamide at 60; for 1 h;Sealed tube;Hantzsch Thiazole Synthesis;
Steps:
2.4 4.2.1. Methyl-4-phenylthiazole (1)30
General procedure: Thioamide 0.75 g (10 mmol) and bromoacetophenone 1.99 g (10 mmol) were dissolved 5 mL of DMF in a 20 mL pressure tube. The reaction mixtures were sealed and heated 1 h at 60 °C in oil bath. The resulting mixture was diluted with saturated aqueous ammonium chloride. The product was extracted with ethyl acetate. The ethyl acetate layer was dried over anhydrous magnesium sulfate, filtered, and concentrated. The product was purified by silica gel column chromatography using hexane:ethyl acetate. 2-Methyl-4-phenyl-thiazole was obtained in 99% yield as a white solid.
References:
Kim, Su Kang;Kim, Ji-Hyun;Park, Young Chul;Kim, Jae Won;Yum, Eul Kgun [Tetrahedron,2013,vol. 69,# 51,p. 10990 - 10995]
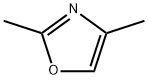
7208-05-1
66 suppliers
$45.00/10mg

541-58-2
261 suppliers
$6.00/1g
77470-53-2
90 suppliers
$19.00/1g

541-58-2
261 suppliers
$6.00/1g