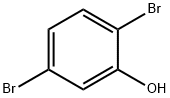
2,5-DIBROMOPHENOL synthesis
- Product Name:2,5-DIBROMOPHENOL
- CAS Number:28165-52-8
- Molecular formula:C6H4Br2O
- Molecular Weight:251.9
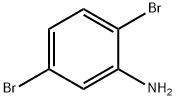
3638-73-1

28165-52-8
General procedure for the synthesis of 2,5-dibromophenol from 2,5-dibromoaniline: Sodium nitrite (65.2 mmol) was added in batches to a solution of trifluoroacetic acid (80 mL) containing 2,5-dibromoaniline (55.8 mmol) at 0 °C. Subsequently, the resulting solution was slowly added to a boiling solution of 50% sulfuric acid (120 mL) containing sodium sulfate (10 g) and the reaction mixture was kept at reflux for 1 hour. Upon completion of the reaction, the product was steam distilled and the distillate was extracted using dichloromethane (2 x 200 mL). The organic layers were combined, dried over sodium sulfate and concentrated to give 2,5-dibromophenol as a yellow solid in 41% yield.
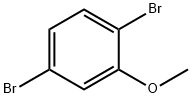
95970-08-4
101 suppliers
$13.00/1g
38865-40-6
0 suppliers
inquiry
Yield:38865-40-6 53.3%
Reaction Conditions:
with aluminum (III) chloride in N,N-dimethyl-formamide at 80 - 100;
Steps:
2.4 (4) Demethylation reaction:
The product of the third step, 2,5-dibromoanisole 1080 g and DMF 5500 mL, was added to the reaction vessel.Stirring, control temperature was added to 688 g of aluminum trichloride in batches at 80-100 °C. The GC tracks until the reaction is over.After the reaction was completed, dichloroethane and water were added, and the mixture was separated.The oil phase was washed with water and separated, and the obtained oil phase was desolvated to obtain a black solid 2,5-dibromophenol 548 g, a content of 98%, and a yield of 53.3%.
References:
CN109320403,2019,A Location in patent:Paragraph 0011; 0015; 0016; 0020

3638-73-1
206 suppliers
$10.00/1g

38865-40-6
0 suppliers
inquiry
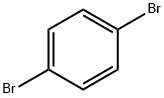
106-37-6
475 suppliers
$9.00/5g

38865-40-6
0 suppliers
inquiry
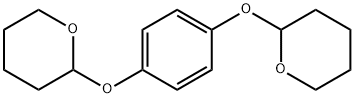
2139-44-8
5 suppliers
inquiry

38865-40-6
0 suppliers
inquiry
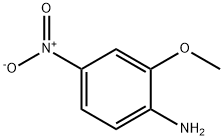
97-52-9
258 suppliers
$10.00/5g

38865-40-6
0 suppliers
inquiry