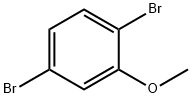
2,5-DIBROMOANISOLE synthesis
- Product Name:2,5-DIBROMOANISOLE
- CAS Number:95970-08-4
- Molecular formula:C7H6Br2O
- Molecular Weight:265.93

67-56-1
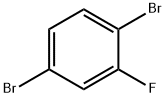
1435-52-5

95970-08-4
The general procedure for the synthesis of 2,5-dibromo-1-methoxybenzene from methanol and 1,4-dibromo-2-fluorobenzene was as follows: to a mixture of 1,4-dibromo-2-fluorobenzene (25.0 g, 98.5 mmol) in a solution of tetrahydrofuran (492 mL) and methanol (40 mL, 984.7 mmol) was added slowly and dropwise potassium tert-butoxide (118.2 mL, 118.2 mmol, 1 M hexane solution). The reaction mixture was stirred at room temperature, then warmed to 70 °C and kept overnight. Upon completion of the reaction, the reaction was quenched with water (50 mL) and the reaction mixture was diluted with ether (400 mL) and subsequently washed once with saturated ammonium chloride solution (300 mL). The aqueous layer was back-extracted with ether (200 mL) and all organic phases were combined and dried over anhydrous sodium sulfate. After filtration, the organic phase was concentrated under reduced pressure to give the crude product. The crude product was purified by fast column chromatography using 5-10% ethyl acetate/hexane as eluent to give 22.0 g (84% yield) of 2,5-dibromo-1-methoxybenzene. The product was analyzed by gas chromatography and the m/z (79Br16Br) was 266 [M]+.
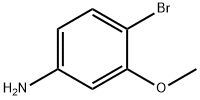
19056-40-7
224 suppliers
$10.00/1g

95970-08-4
104 suppliers
$13.00/1g
Yield: 81%
Reaction Conditions:
Stage #1:4-bromo-3-methoxyaniline with sulfuric acid;sodium nitrite in water at 12;
Stage #2: with hydrogen bromide;copper(I) bromide in water at 40 - 65;
Steps:
2.3 (3) bromine reaction on diazo again
adding 1020 g of 2-bromo-5-aminoanisole to the reaction vessel,Add about 30% sulfuric acid 8380 g and stir.Then, a solution of 455 g of sodium nitrite and 1000 g of water was added dropwise, and the process temperature was controlled at about 12 °C.After the addition is completed, the mixture is stirred for a while to prepare a diazonium salt for use. Add 520 g of cuprous bromide and HBr 1350 g to another reaction flask.The above diazonium salt is added under stirring, and the temperature rises when decomposed, and the controlled temperature is 40 to 65 °C.After the addition, continue to stir, keep the reaction for a period of time, stir, cool, filter,The brown solid 2,5-dibromoanisole was obtained in an amount of 1086 g, and the content was more than 97%, and the yield was 81%.
References:
Changzhou University;Chen Xingquan;Dong Yanmin CN109320403, 2019, A Location in patent:Paragraph 0011; 0013; 0014; 0016; 0018; 0019

67-56-1
790 suppliers
$7.29/5ml-f

1435-52-5
260 suppliers
$7.00/10g

95970-08-4
104 suppliers
$13.00/1g
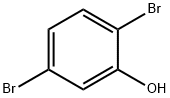
28165-52-8
142 suppliers
$8.00/250mg

77-78-1
319 suppliers
$19.69/1ml

95970-08-4
104 suppliers
$13.00/1g
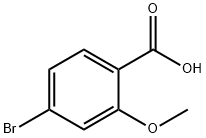
72135-36-5
142 suppliers
$6.00/1g

95970-08-4
104 suppliers
$13.00/1g

28165-52-8
142 suppliers
$8.00/250mg

74-88-4
356 suppliers
$15.00/10g

95970-08-4
104 suppliers
$13.00/1g