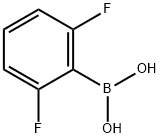
2,6-Difluorophenylboronic acid synthesis
- Product Name:2,6-Difluorophenylboronic acid
- CAS Number:162101-25-9
- Molecular formula:C6H5BF2O2
- Molecular Weight:157.91

121-43-7
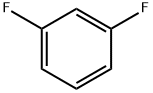
372-18-9

162101-25-9
The general procedure for the synthesis of 2,6-difluorophenylboronic acid from trimethyl borate and 1,3-difluorobenzene was as follows: 22.8 g of 1,3-difluorobenzene (Compound I) and 150 mL of anhydrous ethyl ether were added to a 500 mL dry reaction flask under nitrogen protection and the system was cooled to -78 °C. Slowly 187 mL of 1.6 M n-butyllithium hexane solution was added dropwise to the mixture. After the dropwise addition was completed, the reaction was stirred at -60 to -70 °C for 2 hours. The system was again cooled to -78°C and 60 g of trimethyl borate was slowly added dropwise, and after the dropwise addition was completed, the reaction was stirred at a controlled temperature of -50 to -60°C for 2 hours. The reaction mixture was slowly warmed to room temperature, the reaction was quenched by the addition of 200 mL of water and acidified with 36% hydrochloric acid. After recovering the organic solvent by ascending distillation, the reaction mixture was stirred at 70 to 80 °C for 6 hours. Finally, the system was cooled to crystallization, filtered and washed with cold water to afford 27.0 g of 2,6-difluorophenylboronic acid (Compound II).

121-43-7
382 suppliers
$13.00/25mL

372-18-9
424 suppliers
$9.00/1g

162101-25-9
332 suppliers
$6.00/1g
Yield: 27 g
Reaction Conditions:
Stage #1:1,3-Difluorobenzene with n-butyllithium in diethyl ether;hexane at -70 - -60; for 2 h;Inert atmosphere;
Stage #2:Trimethyl borate in diethyl ether;hexane at -60 - -50; for 2 h;Inert atmosphere;Reagent/catalyst;Temperature;Solvent;
Steps:
1
500 ml of dry reaction flask was replaced with nitrogen, 22.8 g of compound (1), 150 ml of anhydrous ether Liter, and the system was cooled to -78 ° C under nitrogen. The mixture was slowly added dropwise with 187 ml of 1.6 M n-hexane solution of n-butyllithium. After the addition, the reaction was stirred at -60 to -70 ° C 2 hours. The system was re-cooled to -78 ° C, dropping 60 grams of trimethyl borate, dropping finished, the control temperature -50 ~ -60 ° C stirring reaction 2 hours. The reaction was warmed to room temperature, 200 ml of water was added to quench the reaction, and the mixture was acidified with 36% hydrochloric acid. After the organic solvent was recovered by distillation at elevated temperature, the reaction was stirred at 70 to 80 ° C for 6 hours. The system was cooled to crystallize, filtered and washed with water to obtain 27.0 g of the compound (II)
References:
ZHEJIANG ZHONGXIN FU CAI CO LTD/ ZHEJIANG ZHONGXIN CHEMICALS CO., LTD.;YUAN, QILIANG;ZHANG, JIABING;XU, PENGFEI;JIANG, XIANBO;CHEN, YINHAO;WANG, CHAO CN103819401, 2016, B Location in patent:Paragraph 0023; 0066; 0067
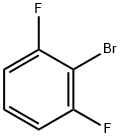
64248-56-2
281 suppliers
$6.00/1g

162101-25-9
332 suppliers
$6.00/1g

64248-56-2
281 suppliers
$6.00/1g

121-43-7
382 suppliers
$13.00/25mL

162101-25-9
332 suppliers
$6.00/1g

372-18-9
424 suppliers
$9.00/1g

162101-25-9
332 suppliers
$6.00/1g