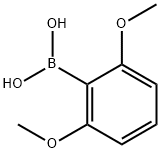
2,6-Dimethoxyphenylboronic acid synthesis
- Product Name:2,6-Dimethoxyphenylboronic acid
- CAS Number:23112-96-1
- Molecular formula:C8H11BO4
- Molecular Weight:181.98

121-43-7
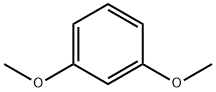
151-10-0

23112-96-1
The general procedure for the synthesis of 2,6-dimethoxyphenylboronic acid from trimethyl borate and m-phenylene dimethyl ether was as follows: 41.5 g (0.3 mol) of 1,3-dimethoxybenzene and 170 g of THF were added to a dry 1L three-neck flask. The reaction system was cooled to -30~-40°C under nitrogen protection and stirred. 240 ml (0.6 mol) of n-butyllithium in hexane solution was added slowly dropwise. After the dropwise addition, the reaction system was slowly warmed to 10~30°C and maintained at this temperature for 1 hour. Subsequently, the system was again cooled to -30~-40 °C and 62.4 g (0.6 mol) of trimethyl borate was slowly added dropwise. After the dropwise addition was completed, the reaction was maintained at this temperature for 1 hour. After the reaction was completed, 148 g of concentrated hydrochloric acid and 150 g of water were added to the system and stirred at 20-30 °C for 1 hour. After completion of the reaction, the solvent was removed by distillation under reduced pressure until no solvent evaporated. The residue was dissolved in 200 g of water and stirred for 20 min, followed by filtration using a Brinell funnel to give 45 g of white solid product in 90.0% yield. The product was analyzed by gas chromatography and the purity was 99.0%.

121-43-7
382 suppliers
$14.00/25mL

151-10-0
467 suppliers
$5.00/10g

23112-96-1
290 suppliers
$6.00/1g
Yield:23112-96-1 90%
Reaction Conditions:
Stage #1: 1,3-Dimethoxybenzenewith n-butyllithium in tetrahydrofuran;hexane at -40 - 30; for 1 h;Inert atmosphere;
Stage #2: Trimethyl borate in tetrahydrofuran;hexane at -40 - -30; for 1 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;hexane;water at 20 - 30; for 1 h;
Steps:
3 Preparation of Compond II
41.5g (0.3mol) 1,3-dimethoxybenzene, 170gTHF are placed into a dry 1L three-necked flask. Under nitrogen protection, stirwhile cooling the system temperature to -30 ~ -40 deg.C. Add dropwise 240ml (0.6mol) n-butyllithium n-hexane solution. Dropping complete. Heat to 10 ~ 30 deg.C and maintain temperature for 1h. After maintaining the temperature, cool the system temperature to -30 ~ -40 deg.C. Add dropwise 62.4g (0.6mol)of trimethyl borate. Dropping complete. Maintain temperature for 1h. Maintain temperature was complete. Add 148g concentrated hydrochloric acid and 150g water. Reaction solution was stirred at 20 ~ 30 deg,C and maintain temperature 1h. Maintain temperature was completed, the reaction solution under reduced pressure until solvent-free solvent removal, the product was added to 200g of water, stirred for 20min, suction filtration using a Buchner funnel to give 45g of white solid, a yield of 90.0%, measured by gas chromatographic purity of 99.0% .
References:
CN105348240,2016,A Location in patent:Paragraph 0059; 0060

151-10-0
467 suppliers
$5.00/10g

23112-96-1
290 suppliers
$6.00/1g

5419-55-6
387 suppliers
$12.00/5g
16932-45-9
147 suppliers
$37.00/1g

23112-96-1
290 suppliers
$6.00/1g

121-43-7
382 suppliers
$14.00/25mL

7732-18-5
507 suppliers
$13.50/100ML

151-10-0
467 suppliers
$5.00/10g

23112-96-1
290 suppliers
$6.00/1g

121-43-7
382 suppliers
$14.00/25mL

16932-45-9
147 suppliers
$37.00/1g

23112-96-1
290 suppliers
$6.00/1g