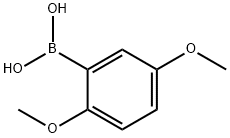
2,5-Dimethoxyphenylboronic acid synthesis
- Product Name:2,5-Dimethoxyphenylboronic acid
- CAS Number:107099-99-0
- Molecular formula:C8H11BO4
- Molecular Weight:181.98

121-43-7
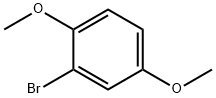
25245-34-5

107099-99-0
In a 1 L round bottom flask, 1-bromo-2,5-dimethoxybenzene (50 g, 230 mmol) was dissolved in tetrahydrofuran (400 ml). After cooling the reaction mixture to -78°C, n-butyllithium (167 ml, 280 mmol) was added slowly and dropwise. The reaction temperature was maintained at -78°C with continuous stirring for 2 hours. Subsequently, trimethyl borate (36 ml, 320 mmol) was added. The reaction mixture was gradually warmed to room temperature and stirred overnight. Upon completion of the reaction, acidification was carried out by slow dropwise addition of 2N HCl solution. Extraction was carried out with deionized water and ethyl acetate and the organic layer was separated and dried over anhydrous magnesium sulfate. The dried organic phase was concentrated in vacuum to give the crude product. Finally, the purified 2,5-dimethoxyphenylboronic acid (20.8 g, 50% yield) was obtained by recrystallization from a solvent mixture of heptane and toluene.

121-43-7
382 suppliers
$14.00/25mL

25245-34-5
234 suppliers
$8.00/5g

107099-99-0
284 suppliers
$6.00/1g
Yield:107099-99-0 50%
Reaction Conditions:
Stage #1: 1-bromo-2,5-dimethoxybenzenewith n-butyllithium in tetrahydrofuran at -78; for 2 h;
Stage #2: Trimethyl borate in tetrahydrofuran at 20;
Steps:
3.1 Synthesis Example 3. Synthesis of Compound 140. Synthesis Example 3-(1). Synthesis of Intermediate 3-a
In a 1-L round-bottom flask reactor, 2-bromo-1,4-dimethoxybenzene (50 g, 230 mmol) and tetrahydrofuran (400 ml) were dissolved. After the mixture was cooled to -78° C., it was added with drops of N-butyl lithium (167 ml, 280 mmol). At the same temperature, the mixture was stirred for 2 hrs before the addition of trimethyl borate (36 ml, 320 mmol). Stirring was conducted overnight at room temperature. After completion of the reaction, 2 N HCl was dropwise added for acidification. Extraction with water and ethyl acetate gave an organic layer which was then dried over magnesium sulfate and concentrated in a vacuum. Recrystallization in heptane and toluene afforded
References:
US2017/18723,2017,A1 Location in patent:Paragraph 0157-0158

121-43-7
382 suppliers
$14.00/25mL

25245-34-5
234 suppliers
$8.00/5g

7732-18-5
507 suppliers
$13.50/100ML

107099-99-0
284 suppliers
$6.00/1g

25245-34-5
234 suppliers
$8.00/5g

107099-99-0
284 suppliers
$6.00/1g

121-43-7
382 suppliers
$14.00/25mL
150-78-7
540 suppliers
$5.00/25g

107099-99-0
284 suppliers
$6.00/1g

150-78-7
540 suppliers
$5.00/25g

107099-99-0
284 suppliers
$6.00/1g