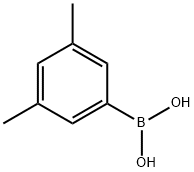
3,5-Dimethylphenylboronic acid synthesis
- Product Name:3,5-Dimethylphenylboronic acid
- CAS Number:172975-69-8
- Molecular formula:C8H11BO2
- Molecular Weight:149.98

67-56-1
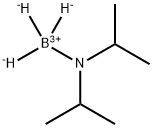
55124-35-1
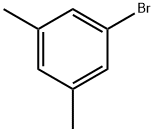
556-96-7

172975-69-8
GENERAL PROCEDURE: A 1 M THF solution (375 μL, 375 μmol) of phenylmagnesium bromide (PhMgBr) was added dropwise to a tetrahydrofuran (THF, 4 mL) solution of diisopropylaminoborane (DIPAB, 863 mg, 7.5 mmol) and magnesium scrapings (Mg, 182 mg, 7.5 mmol) at room temperature. After 10 min of reaction, 30 mL of anhydrous THF was added, followed by 3,5-dimethylbromobenzene (5 mmol). The reaction mixture was cooled to 0 °C and quenched by slow addition of methanol (MeOH, 7 mL). After 1 h of reaction, the volatiles were removed under reduced pressure and the resulting solid was dissolved in a 1N hydrochloric acid/methanol (7:3, v/v) mixture. The reaction was continued for 1 h at room temperature, followed by the addition of ethyl acetate (AcOEt, 100 mL) for extraction. The organic phase was washed sequentially with 1N hydrochloric acid (30 mL) and saturated saline (3 x 30 mL). The organic phase was concentrated under reduced pressure to give the crude product, which was finally purified by recrystallization from water (H2O) to give 3,5-dimethylphenylboronic acid.
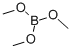
121-43-7
382 suppliers
$13.00/25mL
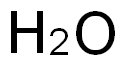
7732-18-5
507 suppliers
$12.69/100ml
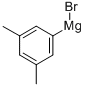
34696-73-6
50 suppliers
$226.00/50mL

172975-69-8
322 suppliers
$9.00/5g
Yield:172975-69-8 74%
Reaction Conditions:
Stage #1:Trimethyl borate;3,5-dimethyphenylmagnesium bromide in tetrahydrofuran at 20;
Stage #2:water with hydrogenchloride in tetrahydrofuran;diethyl ether
Steps:
(3,5-dimethylphenyl)boronic acid
A solution of 3,5-dimethylphenylmagnesium bromide obtained from a solution of190.3 g (1.03 mol) of 1-bromo-3,5-dimethylbenzene in 1000 ml ofTHF and 32 g(1.32 mol, 28% excess) of magnesium turnings was cooled to -78°C, and 104 g (1.0 mol) of trimethylborate was added in one portion. The resulting heterogeneousmixture was stirred overnight at room temperature. The boronic ester washydrolyzed by careful addition of 1200 ml of2 M HCl. 500 ml of diethyl ether wasadded, the organic layer was separated, and the aqueous layer was additionallyextracted with 2 x 500 ml of diethyl ether. The combined organic extract was dried over Na2S04 and then evaporated to dryness to give white mass. The latter wastriturated with 200 ml of hexane, filtered through glass frit (G3), and the precipitatewas dried in vacuo. This procedure gave 114.6 g (74%) of(3,5-dimethylphenyl)boronic acid.Anal. calc. for C8H11B02: C, 64.06; H, 7.39. Found: C, 64.38; H, 7.72. 1H NMR (DMSO-d6): 8 7.38 (s, 2H), 7.00 (s, 1H), 3.44(very br.s, 2H), 2.24 (s, 6H).
References:
BOREALIS AG;AJELLAL, Noureddine;RESCONI, Luigi;IZMER, Vyatcheslav V.;KONONOVICH, Dmitry S.;OSKOBOYNIKOV, Alexander Z.;VIRKKUNEN, Ville WO2018/91684, 2018, A1 Location in patent:Page/Page column 66; 67

67-56-1
790 suppliers
$7.29/5ml-f

55124-35-1
25 suppliers
$60.00/100mg

556-96-7
382 suppliers
$6.00/5g

172975-69-8
322 suppliers
$9.00/5g

556-96-7
382 suppliers
$6.00/5g

172975-69-8
322 suppliers
$9.00/5g
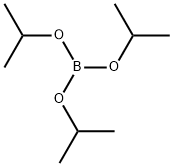
5419-55-6
388 suppliers
$12.00/5g

556-96-7
382 suppliers
$6.00/5g

172975-69-8
322 suppliers
$9.00/5g
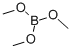
121-43-7
382 suppliers
$13.00/25mL

556-96-7
382 suppliers
$6.00/5g

172975-69-8
322 suppliers
$9.00/5g