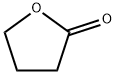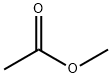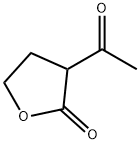
2-Acetylbutyrolactone synthesis
- Product Name:2-Acetylbutyrolactone
- CAS Number:517-23-7
- Molecular formula:C6H8O3
- Molecular Weight:128.13
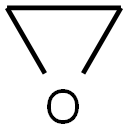
75-21-8
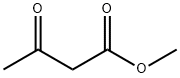
105-45-3

517-23-7
Example 2: Ethylene oxide (EO, 25.0 g, 1.4 eq.) was slowly added to a methanol (MeOH, 144.0 g) solution of methyl acetoacetate (MAA, 46.9 g, 0.40 mol) followed by triethylamine (TEA, 40.7 g, 1.0 eq.) at 5°C. The reaction mixture was warmed up to 60 °C within 0.5 h and maintained at this temperature for 2.5 h. The reaction mixture was then heated up to 60 °C within 0.5 h and maintained at this temperature for 2.5 h. Quantitative GC analysis showed 50.8% conversion of MAA and 72.9% selectivity of α-acetyl-γ-butyrolactone (ABL). Example 4: Ethylene oxide (EO, 35.3 g, 2.0 eq.) was slowly added to a methanol (MeOH, 144 g) solution of methyl acetoacetate (MAA, 47.0 g, 0.40 mol) at 5 °C, followed by triethylamine (TEA, 42.4 g, 1.0 eq.). The reaction mixture was warmed up to 60 °C within 0.5 h and maintained at this temperature for 4.5 h. The reaction mixture was then heated up to 60 °C within 0.5 h and maintained at this temperature for 4.5 h. Quantitative GC analysis showed 67.7% conversion of MAA and 75.0% selectivity of α-acetyl-γ-butyrolactone (ABL). Examples 7-27: The reaction was carried out according to the method of Example 1 by adjusting the amount of ethylene oxide (EO), the optional addition of the catalysts and additives specified in Table 1, and the reaction times specified in Table 1. Table 1 details the reaction conditions, conversion and selectivity data for Examples 7-27. All reactions of Examples 7-26 were carried out at 60°C using 11 equivalents of methanol as solvent and 0.40 equivalents of MAA. Examples 27 and 28, on the other hand, used 0.10 eq. of MAA and 1 eq. of EO at 60 °C, using 11 eq. of methanol as solvent. Example of Example 28: Example of optimal reaction conditions using triethylamine (TEA) as a catalyst: 37 g of methanol was added to a 250 mL autoclave, pressurized with 2-3 bar nitrogen and heated to 65 °C. Subsequently, a solution of MAA (39.9 g, 0.34 mol, 1 eq.) and triethylamine (TEA, 34.4 g, 0.34 mol, 1 eq.) in methanol (27 g) with ethylene oxide (EO, 30.1 g, 0.68 mol, 2 eq.) was added simultaneously via two pumps over 11 min. After 2 h of reaction, analysis by GC showed a selectivity of 77% for ABL and a yield of 52.3% based on the added MAA. The conditions for this series of reactions ranged from 65 to 95 °C, addition times from 9 to 13 min, concentrations of MAA in the solvent from 1.3 to 2.1 mol/L, TEA/MAA molar ratios from 0.7 to 1.3, and EO/MAA molar ratios from 1.7 to 2.2.The results of this series of reactions showed MAA conversions ranging from 65.6 to 85.0%, ABL selectivities of 47.5-77.4% and the yield of ABL based on MAA was 40.4-52.3%.
Yield:517-23-7 96%
Reaction Conditions:
Stage #1: 4-butanolide;acetic acid methyl esterwith sodium methylate in methanol at 45 - 90; under 750.075 Torr; for 10 h;Inert atmosphere;Large scale;
Stage #2: with carbon dioxide in methanol;water at 0 - 35; under 6000.6 Torr; for 0.5 h;Large scale;Temperature;Pressure;Reagent/catalyst;
Steps:
1-15; 1-3 (1) Acylation reaction:
the reaction is carried out in a dry 50L stainless steel reaction tank equipped with stirring, reflux and distillation equipment. Turn on the stirring, replace the reaction tank with nitrogen, add 10.3 kg of methyl acetate, raise the temperature in the reaction tank to 45°C, add 4 kg of γ-butyrolactone and 3.76 kg of sodium methoxide in batches, and keep the system temperature at 45°C. The reaction system is gradually heated to 55°C, the valve of the distillation device is slowly opened, the reflux ratio is controlled to be 3:1, and the mixture of methanol and methyl acetate generated by the reaction is collected about 8kg for rectification, separation and recovery. At the same time, slowly add 7kg of methyl acetate, slowly increase the temperature, the pressure in the tank is 0.1MPa, and the reaction is maintained at 90°C for 10h. The reaction system was monitored by gas chromatography that about 1% of γ-butyrolactone remained.(2) Neutralization reaction: Cool the reaction system in step (1) to 0°C to 5°C, start to replace the gas in the tank with carbon dioxide twice, control the temperature of the system to 35°C, pass carbon dioxide gas into the tank and keep the tank The internal carbon dioxide pressure is 0.8MPa. Start slowly adding 0.7 kg of water dropwise, after the dropwise addition is complete, stir for 30 minutes.(3) Separation: The reaction system in step (2) is filtered by pressure, the solid is washed with methyl acetate and dried to obtain sodium bicarbonate, which is collected as a by-product; the filtrate is first subjected to atmospheric distillation to distill the mixture of methyl acetate and methanol 9.5kg is combined with the distilled product in step (1) to rectify and separate; the remaining liquid in the tank is transferred to a 10L bottle to continue high-vacuum and reduced-pressure distillation under a pressure of less than 1000Pa to obtain 5.73kg of α-acetyl- γ-butyrolactone, its purity and yield are shown in Table 1.
References:
CN111620844,2020,A Location in patent:Paragraph 0053-0123