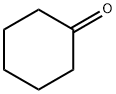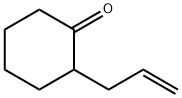
2-Allylcyclohexanone synthesis
- Product Name:2-Allylcyclohexanone
- CAS Number:94-66-6
- Molecular formula:C9H14O
- Molecular Weight:138.21
Yield: 95%
Reaction Conditions:
in 1,4-dioxane at 0; for 4 h;Inert atmosphere;Reflux;
Steps:
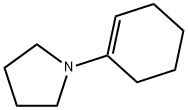
Synthesis of a0-allyl 3 and a0-propargyl 2 cyclohexanones
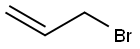
General procedure: To a stirred solution of anhydrous 1,4-dioxane (20 ml) and1-(cyclohex-1-en-1-yl) pyrrolidine 1 (5 g, 33 mmol) at 0 Cequipped with a reflux condenser was added dropwise a mixtureof allylbromide (2.855 ml, 33 mmol) or propargylbromide(3.68 ml, 33 mmol) in anhydrous dioxane (20 mL). The resultantmixture was stirred for 4 h at reflux followed by the addition ofHCl solution (1%, 12 ml) and then by an additional 3 h reflux byremoving 1,4-dioxane. The reaction mixture was washed withdiethyl ether (3 50 mL). The combined organic phase was washedwith brine (50 mL) dried over MgSO4 and vaporated in vacuo. Thecrude products were purified by flash column chromatographywith a mixture of ethylacetate and hexane in suitable ratios. 4.3.1. 2-Allylcyclohexanone 3Colorless oil, (18.05 g, 95% yield). IR mmax (neat, cm1): 1715,1651, 925. 1H NMR (CDCl3, 400 MHz): d 5.72-5.83 (m, 1H), 5.04(d, J = 1.0 Hz, 1H), 4.98-5.01 (m, 1H), 2.50-2.57 (m, 1H), 2.24-2.42 (m, 2H), 2.09-2.16 (m, 2H), 1.94-2.08 (m, 1H), 1.81-1.88(m, 1H), 1.60-1.73 (m, 2H), 1.53 (d, J = 7.0 Hz, 1H), 1.35 (dq,J = 12.0 and 3.5 Hz, 1H). 13C NMR (CDCl3, 100.6 MHz): d 212.4,136.5, 116.2, 50.3, 42.0, 33.8, 33.4, 27.9, 24.9 HRMS (ESI-TOF) forC9H14O [M]+, was calculated as 138.1045 and found to be138.1054.
References:
Özdemirhan, Devrim;Sarıçelik, Özlem [Tetrahedron Asymmetry,2017,vol. 28,# 1,p. 118 - 124]
86950-86-9
0 suppliers
inquiry

94-66-6
86 suppliers
$23.00/1g