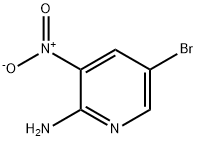
2-Amino-5-bromo-3-nitropyridine synthesis
- Product Name:2-Amino-5-bromo-3-nitropyridine
- CAS Number:6945-68-2
- Molecular formula:C5H4BrN3O2
- Molecular Weight:218.01
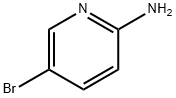
1072-97-5

6945-68-2
General procedure for the synthesis of 2-amino-3-nitro-5-bromopyridine from 2-amino-5-bromopyridine: 2-amino-5-bromopyridine (0.020 mol) was slowly added to concentrated sulfuric acid that was pre-cooled to 0 °C, maintaining the temperature of the reaction system in the 0-5 °C range. Concentrated nitric acid (1.09 mL, 0.024 mol) was slowly added dropwise with continuous stirring, and the rate of dropwise acceleration was controlled to maintain the reaction temperature in the range of 0-5 °C. After the dropwise addition was completed, the reaction mixture was continued to be stirred at 0-5 °C for 1 hour. Subsequently, the reaction system was slowly warmed to room temperature and stirred overnight at room temperature. Upon completion of the reaction, the mixture was carefully poured into crushed ice and the pH was adjusted to 7-8 with 10% aqueous sodium hydroxide solution. the resulting precipitate was collected by filtration, washed well with cold water and finally dried under vacuum at 60 °C overnight to obtain high purity 2-amino-3-nitro-5-bromopyridine.

1072-97-5
705 suppliers
$9.00/1g

6945-68-2
406 suppliers
$9.00/5g
Yield:6945-68-2 71%
Reaction Conditions:
with sulfuric acid;nitric acid at 0 - 20;Inert atmosphere;
Steps:
4.2. General procedure for nitration of 2-aminopyridines 7
General procedure: 2-Aminopyridine 7 (0.020 mol) was added to ice-cold concentrated sulfuric acid and the resulting solution was treated with concentrated nitric acid (1.09 mL, 0.024 mol), which was added slowly so as to maintain the reaction temperature in 0-5 °C range.The resulting solution was stirred at 0-5 °C for 1 h, warmed up to rt and stirred at that temperature overnight. It was then poured over ice and the pH was adjusted to 7-8 with 10% aq NaOH solution. The resulting precipitate was collected by filtration, washed with water and dried at 60 °C overnight to provide analytically pure 2-amino-3-nitropyridines.
References:
Sapegin, Alexander V.;Kalinin, Stanislav A.;Smirnov, Alexey V.;Dorogov, Mikhail V.;Krasavin, Mikhail [Tetrahedron,2014,vol. 70,# 5,p. 1077 - 1083]
4214-75-9
415 suppliers
$9.00/5g

6945-68-2
406 suppliers
$9.00/5g
1072-98-6
715 suppliers
$5.00/1g
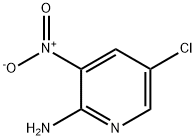
5409-39-2
240 suppliers
$6.00/250mg

6945-68-2
406 suppliers
$9.00/5g
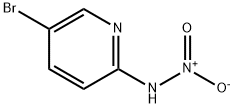
33245-29-3
6 suppliers
inquiry

6945-68-2
406 suppliers
$9.00/5g
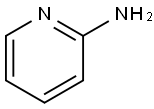
504-29-0
561 suppliers
$5.00/5 g

6945-68-2
406 suppliers
$9.00/5g