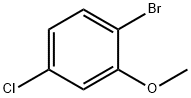
2-BROMO-5-CHLOROANISOLE synthesis
- Product Name:2-BROMO-5-CHLOROANISOLE
- CAS Number:174913-09-8
- Molecular formula:C7H6BrClO
- Molecular Weight:221.48
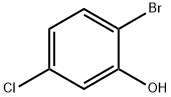
13659-23-9

74-88-4

174913-09-8
2-Bromo-5-chlorophenol (2.85 g, 13.7 mmol) and iodomethane (1.28 mL, 20.6 mmol) were stirred for 2 hours at room temperature in the presence of potassium carbonate (1.89 g, 13.7 mmol), n-BU4NI (50 mg, 0.137 mmol) and N,N-dimethylformamide (8.0 mL). After completion of the reaction, ice water was added and extracted twice with ethyl acetate. The organic phases were combined, washed with brine and dried over anhydrous magnesium sulfate. After filtration, it was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (hexane:ethyl acetate=95:5) to give 2-bromo-5-chloroanisole (2.94 g, 97%) as a colorless oil. Subsequently, oxalyl chloride (1.23 mL, 15.1 mmol) and N,N-dimethylformamide (2 drops) were added to a solution of 4-ethoxybenzoic acid (2.28 g, 13.7 mmol) in chloroform (8 mL) and stirred for 5 hours. The solvent was removed under reduced pressure and the yellow oil obtained was dissolved in chloroform (5 mL). To this solution was added a chloroform solution (10 mL) of 2-bromo-5-chloroanisole (2.94 g, 13.3 mmol), and aluminum chloride (2.07 g, 15.5 mmol) was added in batches at -10 °C. After stirring for 1 h at 5 °C, the mixture was brought to room temperature and continued to stir for 13 h. The reaction was carried out at 5 °C. The reaction mixture was poured into ice water and extracted three times with chloroform. The organic layers were combined and washed sequentially with 1 M hydrochloric acid, water and brine and dried over anhydrous magnesium sulfate. After filtration and concentration under reduced pressure, the residue was purified by NH-type silica gel column chromatography (hexane:ethyl acetate=9:1) to afford (5-bromo-2-chloro-6-methoxyphenyl)(4-ethoxyphenyl)methanone (1.53 g, 31%) as colorless crystals. Finally, Et3SiH (1.62 mL, 10.1 mmol) and BF3*Et2O (0.772 mL, 6.09 mmol) were sequentially added to a chloroform-acetonitrile (1:1; 16 mL) solution of (5-bromo-2-chloro-6-methoxyphenyl)(4-ethoxyphenyl) methanone (1.50 g, 4.06 mmol) at -5 °C. The reaction mixture was slowly warmed to room temperature and stirred for 16 hours. Saturated aqueous sodium carbonate solution was added and extracted with chloroform. The organic layer was washed with brine and dried over anhydrous magnesium sulfate. After filtration, it was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (hexane:ethyl acetate=20:1) to afford the target compound 1-bromo-4-chloro-3-(4-ethoxybenzyl)-6-methoxybenzene (1.48 g, 99%) in the form of a colorless oil.1H NMR (200 MHz, chloroform-d) δ ppm 1.40 (t, J=7.0 Hz, 3H) 3.87 (s, 3H), 3.93 (s, 2H), 4.01 (q, J=7.0 Hz, 2H), 6.77-6.87 (m, 2H), 6.90 (s, 1H), 7.03-7.12 (m, 2H), 7.29 (s, 1H). ei 354 (M+), 356 (M+2), 358 (M+4).

13659-23-9
188 suppliers
$19.00/1g

74-88-4
357 suppliers
$15.00/10g

174913-09-8
215 suppliers
$6.00/1g
Yield:174913-09-8 97%
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;potassium carbonate in N,N-dimethyl-formamide; for 2 h;
Steps:
27
Preparation Example 27 Synthesis of 1-bromo-4-chloro-3-(4-ethoxybenzyl)-6-methoxybenzene; A suspension of 2-bromo-5-chlorophenol (2.85 g, 13.7 mmol; synthesized in reference to International Patent Publication WO0109122), potassium carbonate (1.89 g, 13.7 mmol), n-BU4NI (50 mg, 0.137 mmol), methyl iodide (1.28 mL, 20.6 mmol) and N,N-dimethylformamide (8.0 mL) was stirred for two hours. An iced water was added and the obtained mixture was extracted with ethyl acetate twice. The combined organic phase was washed with brine and dried with anhydrous magnesium sulfate. After the desiccant was filtered off, the residue obtained by evaporating the solvent under reduced pressure was purified by silica gel column chromatography (hexane:ethyl acetate=95:5) to obtain colorless oily 2-bromo-5-chloroanisole (2.94 g, 97%). Then, oxalyl chloride (1.23 mL, 15.1 mmol) and N,N-dimethylformamide (2 drops) were added to 4-ethoxybenzoic acid (2.28 g, 13.7 mmol) in chloroform (8 mL) and stirred for five hours. The yellow oil obtained by evaporating the solvent under reduced pressure was dissolved in chloroform (5 mL). To this solution, a chloroform solution (10 mL) of 2-bromo-5-chloroanisole (2.94 g, 13.3 mmol) was added and then aluminum chloride (2.07 g, 15.5 mmol) was added portion wise at -10°C over five minutes. After stirred at 5°C for one hour, the reaction mixture was to room temperature and stirred for 13 hours. The reaction mixture was poured into an iced water and extracted with chloroform three times. After washed with 1 M hydrochloric acid, water, brine, the combined organic layer was dried with anhydrous magnesium sulfate. After the desiccant was filtered off, the residue obtained by evaporating the solvent under reduced pressure was purified by NH type silica gel column chromatography (hexane:ethyl acetate=9:1) to obtain (5-bromo-2-chloro-6-methoxyphenyl)(4-ethoxyphenyl)methanone (1.53 g, 31%) as a colorless crystal. Then, Et3SiH (1.62 mL, 10.1 mmol) and BF3·Et2O (0.772 mL, 6.09 mmol) were added sequentially to a chloroform - acetonitrile (1:1; 16 mL) solution of (5-bromo-2-chloro-6-methoxyphenyl)(4-ethoxyphenyl)methanone (1.50 g, 4.06 mmol) at -5°C. The reaction mixture was warmed to room temperature and stirred for 16 hours. After the reaction mixture was added with a saturated sodium carbonate aqueous solution and extracted with chloroform, the organic layer was washed with brine and dried with anhydrous magnesium sulfate. After the desiccant was filtered off, the residue obtained by evaporating the solvent under reduced pressure was purified by silica gel column chromatography (hexane:ethyl acetate=20:1) to obtain a colorless oily title compound (1.48 g, 99%). 1H NMR (200 MHz, CHLOROFORM-d) δ ppm 1.40 (t, J=7.0 Hz, 3 H) 3.87 (s, 3 H) 3.93 (s, 2 H) 4.01 (q, J=7.0 Hz, 2 H) 6.77 - 6.87 (m, 2 H) 6.90 (s, 1 H) 7.03 - 7.12 (m, 2 H) 7.29 (s, 1 H). EI 354(M+), 356(M+2), 358(M+4).
References:
EP1845095,2007,A1 Location in patent:Page/Page column 41-42
57479-70-6
177 suppliers
$10.00/1g

174913-09-8
215 suppliers
$6.00/1g

13659-23-9
188 suppliers
$19.00/1g

77-78-1
319 suppliers
$22.00/25g

174913-09-8
215 suppliers
$6.00/1g