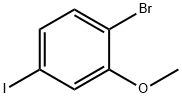
2-BROMO-5-IODOANISOLE synthesis
- Product Name:2-BROMO-5-IODOANISOLE
- CAS Number:755027-18-0
- Molecular formula:C7H6BrIO
- Molecular Weight:312.93
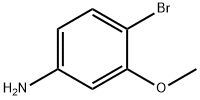
19056-40-7

755027-18-0
4-Bromo-3-methoxyaniline (102 mg, 0.50 mmol) was used as starting material and dissolved in concentrated hydrochloric acid (10 mL) at 0 °C. Subsequently, an aqueous solution of sodium nitrite (NaNO2, 45 mg, 0.65 mmol) (5 mL) was slowly added, the reaction temperature was maintained at 0 °C and the reaction was stirred for 1 hour. Next, an aqueous solution of potassium iodide (KI, 249 mg, 1.5 mmol) (5 mL) was added, and the reaction mixture was gradually warmed to room temperature and stirred overnight. After completion of the reaction, the product was extracted with ethyl acetate. The organic phase was washed sequentially with water and 10% sodium thiosulfate (Na2S2O3) solution, dried over anhydrous magnesium sulfate (MgSO4), filtered and concentrated to give 147 mg of 2-bromo-5-iodoanisole in 94% yield. The product was analyzed by mass spectrometry (ESI), m/e 332 (M + 20)+; nuclear magnetic resonance hydrogen spectrum (1H NMR, 300 MHz, DMSO-d6) δ 7.39 (d, J = 1.87 Hz, 1H), 7.34 (d, J = 8.11 Hz, 1H), 7.24 (dd, J = 8.11, 1.87 Hz, 1H), 3.86 (s 3H).

19056-40-7
220 suppliers
$10.00/1g

755027-18-0
138 suppliers
$9.00/1g
Yield: 100%
Reaction Conditions:
Stage #1:4-bromo-3-methoxyaniline with hydrogenchloride;sodium nitrite in water at 0; for 1 h;
Stage #2: with potassium iodide in water at 0 - 20; for 1 h;
References:
Madden, Katrina S.;Laroche, Benjamin;David, Sylvain;Batsanov, Andrei S.;Thompson, Daniel;Knowles, Jonathan P.;Whiting, Andrew [European Journal of Organic Chemistry,2018,vol. 2018,# 38,p. 5312 - 5322]
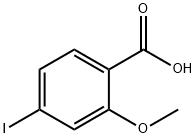
89942-34-7
53 suppliers
$26.00/1g

755027-18-0
138 suppliers
$9.00/1g
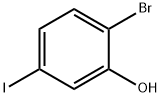
932372-99-1
109 suppliers
$72.00/250 mg

77-78-1
324 suppliers
$19.69/1ml

755027-18-0
138 suppliers
$9.00/1g