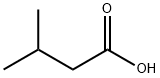
Isovaleric acid
- CAS No.
- 503-74-2
- Chemical Name:
- Isovaleric acid
- Synonyms
- 3-METHYLBUTANOIC ACID;3-METHYLBUTYRIC ACID;ISOPENTANOIC ACID;isopentanoic;3-methylbutyrate;Isovalerianic acid;3-Methybutyric Acid;Butanoicacid,3-methyl-;FEMA 3102;Aroma Name
- CBNumber:
- CB8729604
- Molecular Formula:
- C5H10O2
- Molecular Weight:
- 102.13
- MDL Number:
- MFCD00002726
- MOL File:
- 503-74-2.mol
- MSDS File:
- SDS
| Melting point | -29 °C (lit.) |
|---|---|
| Boiling point | 175-177 °C (lit.) |
| Density | 0.925 g/mL at 20 °C (lit.) |
| vapor pressure | 0.38 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 3102 | ISOVALERIC ACID |
| Flash point | 159 °F |
| storage temp. | Store below +30°C. |
| solubility | 48g/l |
| form | Liquid |
| pka | 4.77(at 25℃) |
| color | Clear colorless to slightly yellow |
| Specific Gravity | 0.928 (20/20℃) |
| PH | 3.92(1 mM solution);3.4(10 mM solution);2.89(100 mM solution); |
| Odor | at 1.00 % in propylene glycol. sour stinky feet sweaty cheese tropical |
| Odor Type | cheesy |
| biological source | synthetic |
| explosive limit | 1.5-6.8%(V) |
| Water Solubility | 25 g/L (20 ºC) |
| Merck | 14,5231 |
| JECFA Number | 259 |
| BRN | 1098522 |
| Dielectric constant | 2.6(20℃) |
| LogP | 1.7 at 25℃ |
| Substances Added to Food (formerly EAFUS) | ISOVALERIC ACID |
| FDA 21 CFR | 172.515 |
| CAS DataBase Reference | 503-74-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 1BR7X184L5 |
| NIST Chemistry Reference | Butanoic acid, 3-methyl-(503-74-2) |
| EPA Substance Registry System | Isovaleric acid (503-74-2) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314 | |||||||||
| Precautionary statements | P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363 | |||||||||
| Hazard Codes | C,T | |||||||||
| Risk Statements | 34-24-22 | |||||||||
| Safety Statements | 26-36/37/39-45-38-28A | |||||||||
| RIDADR | UN 3265 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | NY1400000 | |||||||||
| F | 13 | |||||||||
| Autoignition Temperature | 824 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2915 60 90 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| Hazardous Substances Data | 503-74-2(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 i.v. in mice: 1120±30 mg/kg (Or, Wretlind) | |||||||||
| NFPA 704 |
|
Isovaleric acid price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W310212 | Isovaleric Acid natural, ≥98%, FG | 503-74-2 | 1kg | $191 | 2024-03-01 | Buy |
| Sigma-Aldrich | W310212 | Isovaleric Acid natural, ≥98%, FG | 503-74-2 | 5kg | $683 | 2024-03-01 | Buy |
| Sigma-Aldrich | 129542 | Isovaleric acid 99% | 503-74-2 | 100ml | $33.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 129542 | Isovaleric acid 99% | 503-74-2 | 500ml | $69.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | W310212 | Isovaleric Acid natural, ≥98%, FG | 503-74-2 | 100g | $74.9 | 2023-01-07 | Buy |
Isovaleric acid Chemical Properties,Uses,Production
Description
Isovaleric acid has a characteristic disagreeable odor. It is extremely penetrating and persistent with a sour taste. May be synthesized by oxidation of isoamyl alcohol or isovaleric aldehyde.
Chemical Properties
Isovaleric acid has a characteristic disagreeable, rancid, cheese-like odor. It is extremely penetrating and persistent with a sour taste. May consist of one or a mixture of isomers or n-pentanoic acid and/or 2- or 3-methyl butanoic acid. Consumption: Annual: 1850.00 lb
Chemical Properties
clear colorless to slightly yellow liquid
Occurrence
Of the three possible isomers of n-valeric acid, only isovaleric acid has extensive application in flavoring; originally reported in seal and dolphin fat; subsequently isolated from valerian. Also reported found in the essential oils of cypress, citronella, laurel leaves, cajeput, Cymbopogon javanensis, hops, Persea pubescens, geranium, American peppermint, spearmint, rosemary, lemongrass, Eucalyptus goniocalyx and other spp., tobacco, Monarda fistulos, Thymus mastichina, Artemisia frigida, and probably in lavender; reported among the constituents of petitgrain lemon. Also reported found in many foods including apple, currants, guava, grapes, papaya, peach, pineapple, raspberry, strawberry, potato, bell pepper, vinegar, breads, many cheeses, fish, chicken, lamb, hop oil, beer, cognac, whiskies, cider, sherry, grape wines, rum, cocoa, tea, coffee, honey, soybean, passion fruit, mushrooms, marjoram, plum, brandy, starfruit, trassi, rice, jackfruit, sake, sukiyaki, buckwheat, corn oil, cashew apple, malt, wort, Bourbon vanilla, shrimp, mussels, cherimoya, Cape gooseberry and Chinese quince frui
Uses
Isovaleric acid is used extensively as a flavoring ingredient in nonalcoholic beverages and in foods such as ice cream, candy, baked goods, and cheese, as a fragrance ingredient in perfumes, and as a chemical intermediate in the manufacture of sedatives and other pharmaceutical products. It is also used as an extractant of mercaptans from petroleum hydrocarbons, a vinyl stabilizer, and as an intermediate in the manufacture of plasticizers and synthetic lubricants.
Uses
A molecular entity capable of donating a hydron to an acceptor (Bronsted base). It is a favorable carbon source for cell growth. Moreover, it is a promising odor indicator.
Uses
In flavors, perfumes, manufacture of sedatives.
Definition
ChEBI: A C5, branched-chain saturated fatty acid.
Preparation
By oxidation of isoamyl alcohol or isovaleric aldehyde
Aroma threshold values
Detection: 190 ppb to 2.8 ppm
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 30, p. 2787, 1982 DOI: 10.1248/cpb.30.2787
Tetrahedron Letters, 23, p. 3135, 1982 DOI: 10.1016/S0040-4039(00)88578-0
General Description
Isovaleric acid is a colorless liquid with a penetrating odor. Isovaleric acid is slightly soluble in water. Isovaleric acid is corrosive to metals and to tissue.
Air & Water Reactions
Isovaleric acid is slightly soluble in water.
Reactivity Profile
Isovaleric acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Isovaleric acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Hazard
Strong irritant to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Isovaleric acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Xiangduo Industry Co., Ltd. | +86-15981848961 +86-15981848961 | sales@xiangduochem.com | China | 497 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12806 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531153977 | allison@yan-xi.com | China | 5854 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5868 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2472 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3005 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3692 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 | info@deshangchem.com | China | 662 | 58 |
| JINING XINHE CHEMICAL CO., LTD | +8615318402391 | sales@xinhepharma.com | China | 809 | 58 |
View Lastest Price from Isovaleric acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-04-30 | Isovaleric acid
503-74-2
|
US $29.00-59.00 / mg | 98% | 10g | TargetMol Chemicals Inc. | ||
 |
2025-04-28 | Isovaleric acid
503-74-2
|
US $0.00-0.00 / kg | 25kg | 99%min | 1000kg | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2025-04-27 | Isovaleric acid
503-74-2
|
US $100.00-75.00 / kg | 1kg | 99% | 5000Ton | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD |
-

- Isovaleric acid
503-74-2
- US $29.00-59.00 / mg
- 98%
- TargetMol Chemicals Inc.
-

- Isovaleric acid
503-74-2
- US $0.00-0.00 / kg
- 99%min
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Isovaleric acid
503-74-2
- US $100.00-75.00 / kg
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD