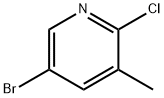
2-Chloro-3-methyl-5-bromopyridine synthesis
- Product Name:2-Chloro-3-methyl-5-bromopyridine
- CAS Number:29241-60-9
- Molecular formula:C6H5BrClN
- Molecular Weight:206.47
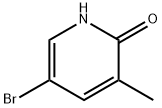
89488-30-2

29241-60-9
Example 11B Synthesis of 5-bromo-2-chloro-3-methylpyridine: The product of Example 11A, 5-bromo-3-methylpyridin-2-one (4.1 g, 22.18 mmol), was dissolved in DMF (40 mL) and phosphoryl chloride (10 g, 65.4 mmol) was added drop-wise at 0 °C. The reaction mixture was heated at 120 °C for 2 hours. Upon completion of the reaction, the mixture was cooled to room temperature and carefully poured into an ice/water mixture. The mixture was adjusted to alkaline with NH4OH, the precipitate was collected by filtration and the filter cake was washed with ice water. The resulting solid was dissolved in dichloromethane (100 mL), the organic layer was washed with brine and dried with anhydrous MgSO4. The dried solution was filtered through a silica pad and the filtrate was concentrated to give 2-chloro-3-methyl-5-bromopyridine as a white solid (3.48 g, 78% yield). The product was characterized by 1H NMR (CDCl3, 300MHz): δ 2.39 (s, 3H), 7.70 (m, 1H), 8.31 (d, J = 3Hz, 1H).

89488-30-2
252 suppliers
$7.00/1g

29241-60-9
280 suppliers
$6.00/1g
Yield:29241-60-9 78%
Reaction Conditions:
with trichlorophosphate in dichloromethane;N,N-dimethyl-formamide
Steps:
11.B 5-bromo-2-chloro-3-methylpyridine
EXAMPLE 11B 5-bromo-2-chloro-3-methylpyridine The product from Example 11A (4.1 g, 221.8 mmol) in DMF (40 mL) was treated with phosphorus oxychloride (10 g, 65.4 mmol) dropwise at 0° C. After heating at 120° C. for 2 hours, the mixture was cooled and poured onto ice/water. The mixture was made basic with NH4OH, filtered, and the filtercake washed with ice water. The obtained solid was dissolved in dichloromethane (100 mL), washed with brine, and dried (MgSO4). The dried solution was filtered through a pad of silica using dichloromethane and the filtrate concentrated to provide the title compound as a white solid (3.48 g, 78% yield): 1H NMR (CDCl3, 300 MHz) δ 2.39 (s, 3H), 7.70 (m, 1H), 8.31, d, J=3 Hz, 1H).
References:
Schrimpf, Michael R.;Tietje, Karin R.;Toupence, Richard B.;Ji, Jianguo;Basha, Anwer;Bunnelle, William H.;Daanen, Jerome F.;Pace, Jennifer M.;Sippy, Kevin B. US2002/19388, 2002, A1
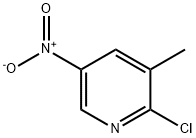
22280-56-4
277 suppliers
$6.00/1g

29241-60-9
280 suppliers
$6.00/1g
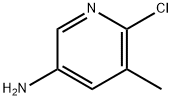
38186-82-2
194 suppliers
$6.00/1g

29241-60-9
280 suppliers
$6.00/1g