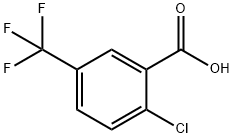
2-Chloro-5-(trifluoromethyl)benzoic acid synthesis
- Product Name:2-Chloro-5-(trifluoromethyl)benzoic acid
- CAS Number:657-06-7
- Molecular formula:C8H4ClF3O2
- Molecular Weight:224.56

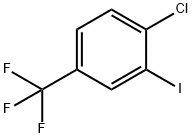
124-38-9
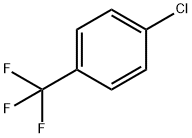
98-56-6

657-06-7
Under nitrogen protection, n-BuLi (2.5 M hexane solution, 22 mL, 55 mmol) was added dropwise to a stirred solution of 4-chlorobenzotrifluoride (9.177 g, 50.8 mmol) and TMEDA (6.3030 g, 54.2 mmol) in THF (89 mL) over a period of 8 min, keeping the reaction temperature at -78 °C. After 52 min of reaction, the reaction solution was transferred via cannula to a vessel containing dry ice (~200 g) within 20 min. Care was taken to keep the cannula fully lagged to prevent rapid darkening of the anion solution on slight warming. The mixture was slowly warmed to room temperature and the solvent was evaporated (temperature controlled at <30°C) to give an orange to yellow solid. The solid was dissolved in water (70 mL) and the aqueous layer was washed with ether (3 x 30 mL). Subsequently, the aqueous layer was adjusted to pH 1 with acid and extracted with DCM (3 x 30 mL). The organic layers were combined, evaporated to dryness (<30°C), and the residue was dissolved in refluxed hexane, hot-filtered and cooled to 4°C for overnight crystallization. The first sand-colored solids (4.5243 g) were obtained by vacuum filtration. The mother liquor was concentrated to half volume to give a second batch of lemon yellow crystals (2.2998 g). The two batches were dried under vacuum to give 4.5030 g and 2.2093 g, respectively, for a total yield of 6.712 g (59%).LCMS analysis showed a purity of 96.3 Apercent (at 254 nm) for the first batch and 95.1 Apercent for the second batch, [M + H] + 225.0, at an Rt = 5.94 min. No starting material was detected.
Yield:657-06-7 59%
Reaction Conditions:
Stage #1: 4-chlorobenzotrifluoridewith n-butyllithium;N,N,N,N,-tetramethylethylenediamine in tetrahydrofuran;hexanes at -78; for 1 h;Inert atmosphere;
Stage #2: carbon dioxide in tetrahydrofuran;hexanes; for 0.333333 h;
Stage #3: in water; pH=1;Acidic aqueous solution;
Steps:
Preparation of 2-chloro-5-trifluoromethylbenzoic acid [Show Image]n-BuLi (2.5M in hexanes, 22 mL, 55 mmol) was added over 8 minutes to a stirred solution of 4-chlorobenzotrifluoride (9.177 g, 50.8 mmol) and TMEDA (6.3030 g, 54.2 mmol) in THF (89 mL) at -78°C under nitrogen. After 52 min the solution was transferred via cannula into dry ice (~200g) over 20 min. Care was required to sufficiently lag the cannula as the anion solution was found to darken rapidly on slight warming. The mixture was allowed to warm to ambient temperature with stirring and was evaporated (<30°C) to give and orange/yellow solid. The solid was dissolved in water (70 mL) and washed with diethyl ether (3x30 mL). The aqueous layer was acidified to pH 1 and extracted with DCM (3x30 mL). The organic layer was evaporated to dryness (< 30°C) and the residue was dissolved in refluxing hexanes, hot filtered and crystallised by cooling to 4°C overnight. The sandy coloured solid was isolated by vacuum filtration (4.5243 g). The mother liquors were concentrated by half and a second crop of lemon yellow crystals was obtained (2.2998 g). The products were dried under vacuum to give 4.5030 g and 2.2093 g respectively, giving a combined yield of 6.712 g (59 %). LCMS: Rt = 5.94 min, first crop = 96.3 A% (at) 254 nm, second crop 95.1 A%, [M+H]+ 225.0, with no starting material detected.
References:
EP2468746,2012,A1 Location in patent:Page/Page column 6

98-56-6
410 suppliers
$11.00/25g

657-06-7
187 suppliers
$7.00/1g
121-50-6
343 suppliers
$5.00/5g

657-06-7
187 suppliers
$7.00/1g