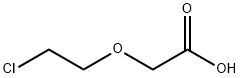
2-CHLOROETHOXY ACETIC ACID synthesis
- Product Name:2-CHLOROETHOXY ACETIC ACID
- CAS Number:14869-41-1
- Molecular formula:C4H7ClO3
- Molecular Weight:138.55
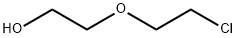
628-89-7

14869-41-1
The general procedure for the synthesis of 2-(2-chloroethoxy)acetic acid from 2-chloroethoxyethanol was as follows: ethylene glycol mono-2-chloroethyl ether (10 g, 0.08 mol) was mixed with a 30% aqueous hydrogen peroxide solution (22.8 g, 0.2 mol), followed by the addition of sodium sulfate dihydrate (0.53 g, 1.6 mmol) and tri-octylmethyl ammonium sulfate (0.75 g, 1.6 mmol). The reaction mixture was stirred at 90°C for 4 hours. Upon completion of the reaction, the reaction was quenched by the addition of aqueous sodium thiosulfate, and then the mixture was extracted with ethyl acetate (50 mL). The organic layers were combined, washed sequentially with water and saturated saline, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give an oily residue (9 g). The residue was diluted with ether and filtered to remove insoluble impurities. The filtrate was extracted by partitioning with the addition of aqueous sodium bicarbonate. The aqueous layer was washed with ether, acidified to pH 2-3 with dilute hydrochloric acid and extracted with ethyl acetate. The organic layers were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give 2-(2-chloroethoxy)acetic acid crude (2.14 g, 19.3% yield) as an oil.IR (neat): ν = 3410 (O-H), 1727 (C=O), 1123, 1044 cm?1 .

628-89-7
385 suppliers
$14.00/5g

14869-41-1
145 suppliers
$23.00/100mg
Yield:14869-41-1 72%
Reaction Conditions:
Stage #1:2-(2-Chloroethoxy)ethanol with calcium hypochlorite;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;sodium hydrogencarbonate in dichloromethane;water at 20; for 0.5 h;
Stage #2: with sodium metabisulfite in dichloromethane;water
Steps:
2 2-(2-chloroethoxy)acetic acid (X)
150 ml dichloromethane, 60 ml tap water, 30.0 ml 2-(2-chloroethoxy)ethanol (XI), 28.52g sodium hydrogencarbonate, and Q.22g TEMPO catalyst are measured to a sulfonating flask equipped with condenser, thermometer, dropping funnel. Then at roo temperature 240.0 ml solution of 55.62g calcium-hypochlorite in tap water is added from the dropping funnel. The mixture is stirred for a further half an hour, then 1.34g sodium metabisulfite is added to the reaction mixture, the two phases are separated in a separation funnel, then the aqueous phase is washed with 2x10 ml dichloromethane. Then the aqueous phase is acidified to pH=T-2 with 34% hydrochloric acid solution. The obtained aqueous solution is extracted with 5x50 ml methyl isobutyl ketone. The combined organic extract is washed with 1x20 ml saturated salt solution, then at a reduced pressure evaporated to constant mass. (0073) The mass of the obtained pale yellow oil : 28.4g, yield 72%.
References:
RICHTER GEDEON NYRT.;SZABÓ, Tamás;NEU, József;GARADNAY, Sándor WO2019/138362, 2019, A1 Location in patent:Page/Page column 9-10
123-91-1
710 suppliers
$16.00/25g

14869-41-1
145 suppliers
$23.00/100mg
17229-14-0
151 suppliers
$25.00/10g

14869-41-1
145 suppliers
$23.00/100mg
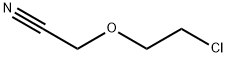
31250-08-5
67 suppliers
inquiry

14869-41-1
145 suppliers
$23.00/100mg

111-46-6
1119 suppliers
$5.00/25g

14869-41-1
145 suppliers
$23.00/100mg