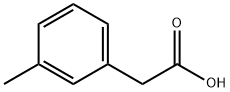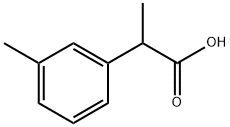
2-(m-Tolyl)propanoicacid synthesis
- Product Name:2-(m-Tolyl)propanoicacid
- CAS Number:73721-06-9
- Molecular formula:C10H12O2
- Molecular Weight:164.2
Yield:73721-06-9 100%
Reaction Conditions:
Stage #1: m-methylphenylacetic acidwith lithium diisopropyl amide in tetrahydrofuran at -20 - -10; for 0.5 h;Inert atmosphere;
Stage #2: methyl iodide in tetrahydrofuran at -20 - 0; for 0.5 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
8.1.1.a; 8.1.2.b 8.1.2. Preparation of methylphenylpropionic acid (13)
b.) base: Lithium diisoyrovylamide (LDA ) Preparation of LDA solution: 187 mL of diisopropylamine was dissolved in 300 mL of anhydrous tetrahydrofuran. Under an inert atmosphere, the solution was cooled to -20 °C, then 511 mL of a 2.5 M butyl lithium solution was added. The reaction mixture was stirred for 2 hours at -10 °C. Alkylatiorr. 80 g of methylphenylacetic acid (12) was dissolved in 800 mL of anhydrous tetrahydrofuran. Under an inert atmosphere, the solution was cooled to -20 °C, then, while maintaining the temperature, the prepared LDA solution was added thereto. The reaction mixture was stirred at -10 °C for 30 minutes, then 40 mL of methyl iodide was added at -20 to -10 °C. After the addition, the reaction mixture was stirred at 0 °C for 30 minutes. The reaction mixture was then quenched with 1200 mL of a 2 M sodium hydrogen sulphate solution, and after vigorous mixing the phases were separated. The aqueous phase was extracted with /c/7-butyl methyl ether. The combined organic phase was washed with saturated sodium chloride solution twice, and at the first washing step, 1.52 g of sodium pyrosulphite was also added to the mixture. The organic layer was dried over sodium sulphate, the desiccant was filtered off, washed and the filtrate was evaporated under reduced pressure. Yield: 87.5 g (100%), orange liquid.
References:
WO2021/123848,2021,A1 Location in patent:Page/Page column 57-58

64-18-6
1119 suppliers
$22.12/250ML
100-80-1
128 suppliers
$27.00/1g

73721-06-9
23 suppliers
inquiry