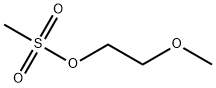
2-METHOXYMETHYL METHANSULFONATE synthesis
- Product Name:2-METHOXYMETHYL METHANSULFONATE
- CAS Number:16427-44-4
- Molecular formula:C4H10O4S
- Molecular Weight:154.18

109-86-4

124-63-0

16427-44-4
GENERAL STEPS: To a cooled solution of 2-methoxyethanol (0.8 mL) in dichloromethane (50 mL) at 0°C, methylsulfonyl chloride (1.3 mL) and triethylamine (2 mL) were added slowly and dropwise. After 5 minutes of reaction, the ice bath was removed and the reaction mixture was gradually warmed to room temperature with continuous stirring for 1 hour. Upon completion of the reaction, the reaction mixture was washed sequentially with 1.0 N NaOH solution and saturated saline, followed by drying with anhydrous magnesium sulfate and concentration under reduced pressure to give 2-methoxyethyl methane sulfonate as an oil in quantitative yield. Mass spectrum (ESI+) m/z 155.1 ([M+H]+), molecular formula C4H10O4S. 2-Methoxyethyl methane sulfonate (1.54 g) was dissolved in 2-butanone (150 mL) and cesium carbonate (6.50 g) and 3-fluoro-4-nitrophenol (1.52 g) were added. The reaction mixture was refluxed for 19 h. After cooling, it was filtered, concentrated under reduced pressure and purified by silica gel column chromatography (eluent: ethyl acetate/heptane) to give the target product in 98% yield.1H NMR (400 MHz, DMSO-d6) δ 7.95, 6.82, 6.67, 4.25, 3.92, 3.68, 3.33, 3.31. 2-Methoxy-4-(2-methoxyethoxy)-1-nitrobenzene (2.0 g) was dissolved in methanol (200 mL) and 10% Pd/C (1.0 g) and HCl (1.8 g) were added. The reaction was shaken at 40 psi hydrogen pressure for 15 minutes. After completion of the reaction, the reaction mixture was filtered and the filtrate was crystallized by ethanol/ether to give 2-methoxy-4-(2-methoxyethoxy)aniline hydrochloride in 76% yield. Mass spectrum (ESI+) m/z 198.1 ([M+H]+), molecular formula C10H15NO3. Example 156 was prepared from 2-methoxy-4-(2-methoxyethoxy)aniline hydrochloride and 2-isocyanato-5-(trifluoromethyl)-1,3,4-thiadiazole according to Method B in 50% yield. High resolution mass spectrometry (HRMS, ESI) calculated value C14H15N4O4SF3+H 393.0844 and measured value 393.0838.
616-47-7
763 suppliers
$10.00/100g

109-86-4
574 suppliers
$11.00/25g

124-63-0
0 suppliers
$10.00/5g

16427-44-4
95 suppliers
$15.00/1g
35487-17-3
57 suppliers
$56.00/25g
Yield: 98%
Reaction Conditions:
in dichloromethane at 10 - 20; for 4 h;
Steps:
1.1
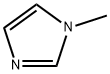
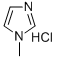
Example 1-(1); Synthesis of Ethylene Glycol Monomethyl Ether Methanesulfonate; 1-Methylimidazole (50 g) and methanesulfonyl chloride (66 g) were mixed in dichloromethane in a 500 mL two-bulb flask. After adding ethylene glycol monomethyl ether (42 g) dropwise at 10° C., the mixture was stirred at room temperature for 4 hours. After the reaction was completed, water was added. After stirring for 10 minutes, the solvent layer in which the product was dissolved was separated from the aqueous layer in which the byproduct was dissolved. The product was yielded by removing the solvent at room temperature using an evaporator (yield: 98%), and the byproduct 1-methylimidazolium chloride was recovered as 1-methylimidazole using 40 wt % NaOH aqueous solution and used again (yield: 95%).
References:
Korea Institute of Science and Technology US2012/83642, 2012, A1 Location in patent:Page/Page column 4