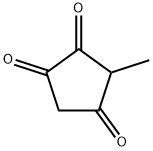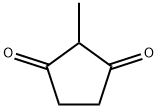
2-Methyl-1,3-cyclopentanedione synthesis
- Product Name:2-Methyl-1,3-cyclopentanedione
- CAS Number:765-69-5
- Molecular formula:C6H8O2
- Molecular Weight:112.13
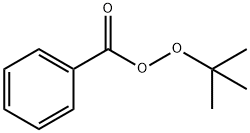
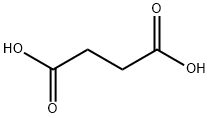
614-45-9
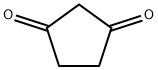
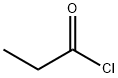
3859-41-4

765-69-5
The general procedure for the synthesis of 2-methyl-1,3-cyclopentanedione from 1,3-cyclopentanedione and tert-butyl peroxybenzoate was as follows: 1,3-cyclopentanedione (0.098 g, 1 mmol), tert-butyl peroxybenzoate (0.58 g, 3 mmol), CuCl (0.01 g, 0.1 mmol), and 2 mL of acetic acid were added to a reaction flask, and the reaction was carried out at 120 °C Reaction at 120°C. The progress of the reaction was monitored by thin layer chromatography (TLC) until the reaction was complete. After completion of the reaction, the crude product was purified by column chromatography (eluent ratio of petroleum ether:ethyl acetate = 40:1) to afford the target product 2-methyl-1,3-cyclopentanedione in 68% yield.
Yield:765-69-5 68%
Reaction Conditions:
with acetic acid;copper(l) chloride at 120;
Steps:
24 Example 24: Preparation of 2-methyl-1,3-cyclopentanedione
In order to 1,3-cyclopentanedione, tert-butyl peroxybenzoate is used as a raw material, the reaction steps are as follows:In the reaction flask by adding 1,3-cyclopentanedione (0.098g, 1mmol), tert-butyl peroxybenzoate (0.58g, 3mmol), CuCl (0.01g, 0 . 1mmol) and 2 ml acetic acid, 120 °C reaction;TLC until the complete end tracking of the reaction;After the reaction the crude product by column chromatography (petroleum ether: ethyl acetate = 40:1), to obtain the target product (yield 68%).
References:
Soochow University;Zou, Jianping;Zhou, Shaofang;Zhang, Peizhi;Zhang, Guoyu;Zhou, Pengjun;Li, Chengkun CN105254483, 2016, A Location in patent:Paragraph 0044
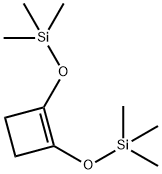
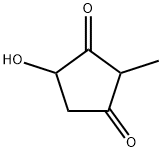
17082-61-0
138 suppliers
$20.00/250mg
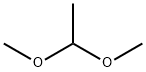
534-15-6
162 suppliers
$26.80/25g

765-69-5
255 suppliers
$6.00/5g
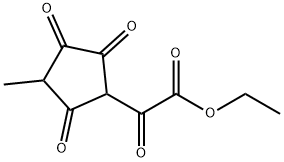
781-38-4
5 suppliers
inquiry

765-69-5
255 suppliers
$6.00/5g