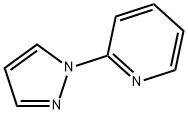
2-PYRAZOL-1-YL-PYRIDINE synthesis
- Product Name:2-PYRAZOL-1-YL-PYRIDINE
- CAS Number:25700-11-2
- Molecular formula:C8H7N3
- Molecular Weight:145.16
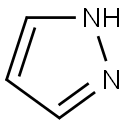
288-13-1
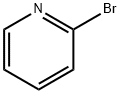
109-04-6

25700-11-2
General procedure for the synthesis of 2-pyrazole-1-pyridine (Pyr-N-PzH) from pyrazole and 2-bromopyridine: To a 250 mL single-necked round-bottomed flask were added 15.0 g (95 mmol) of 2-bromopyridine, 25.0 g (367 mmol, 3.86 equiv) of pyrazole and 45 mL of xylene. The reaction mixture was heated to reflux for 8 hours and subsequently cooled to room temperature. The resulting mixture was dissolved in dichloromethane and the organic layer was washed four times with 250 mL of water (until pyrazole was not detected by gas chromatography). The organic layer was dried over anhydrous magnesium sulfate, filtered and rotary evaporated to give 12.0 g (82.6 mmol, 87% yield) of the target product as a white solid. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 8.59 (m, 1H, pyridinyl 6-H), 8.39 (s, 1H, pyrazol-5-H), 7.90 (d, 1H, pyridinyl 3-H), 7.80 (m, 1H, pyridinyl 4-H), 7.73 (s, 1H, pyrazol 3-H), 7.15 (s, 1H, pyridinyl -5-H), 6.45 (m, 1H, pyrazole-4-H).

288-13-1
455 suppliers
$10.00/1g
1120-90-7
304 suppliers
$5.00/250mg

25700-11-2
87 suppliers
$23.00/250mg
Yield: 94%
Reaction Conditions:
with copper(l) iodide;manganese(II) fluoride;(1R,2R)-1,2-diaminocyclohexane;potassium hydroxide in water at 100; for 48 h;
Steps:
General procedure for N-arylation of nitrogen nucleophiles
General procedure: The N-nucleophile (1.47 mmol), CuI (Sigma-Aldrich, 99.999% purity, 0.147 mmol), MnF2 (Sigma-Aldrich, 98% purity, 0.441 mmol), KOH (2.94 mmol), the aryl halide (2.21 mmol), trans-1,2-diaminocyclohexane (0.294 mmol) and water (0.75 mL) were added to a reaction vial and a screw cap was fitted to it. The reaction mixture was stirred under air in a closed system at 60C for 24 h. After cooling to room temperature, the mixture was diluted with dichloromethane and filtered through a pad of Celite. The combined organic extracts were dried with anhydrous Na2SO4 and the solvent was removed under reduced pressure. The crude product was purified by silica-gel column chromatography to afford the N-arylated product. The identity and purity of known products was confirmed by 1H and 13C NMR spectroscopic analysis.
References:
Teo, Yong-Chua;Yong, Fui-Fong;Lim, Gina Shiyun [Tetrahedron Letters,2011,vol. 52,# 52,p. 7171 - 7174] Location in patent:supporting information; experimental part

288-13-1
455 suppliers
$10.00/1g

109-04-6
528 suppliers
$6.00/25g

25700-11-2
87 suppliers
$23.00/250mg

288-13-1
455 suppliers
$10.00/1g
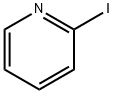
5029-67-4
300 suppliers
$6.00/1g

25700-11-2
87 suppliers
$23.00/250mg

288-13-1
455 suppliers
$10.00/1g
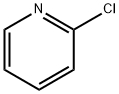
109-09-1
0 suppliers
$10.60/1gm:

25700-11-2
87 suppliers
$23.00/250mg
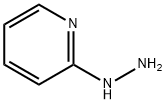
4930-98-7
254 suppliers
$5.00/1g
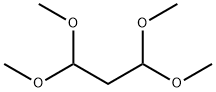
102-52-3
372 suppliers
$5.00/10g

25700-11-2
87 suppliers
$23.00/250mg