2-(Pyridin-2-yl)-1-(p-tolyl)ethanone synthesis
- Product Name:2-(Pyridin-2-yl)-1-(p-tolyl)ethanone
- CAS Number:72076-59-6
- Molecular formula:C14H13NO
- Molecular Weight:211.26
Yield:72076-59-6 73 %Spectr.
Reaction Conditions:
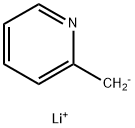
Stage #1: α-picolinewith n-butyllithium in tetrahydrofuran;hexane;water at 0 - 20; pH=1; for 4 h;
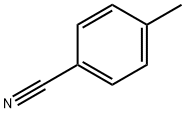
Stage #2: para-methylbenzonitrile in tetrahydrofuran;hexane;water at 0 - 20; for 6 h;
Stage #3: with sulfuric acid in tetrahydrofuran;hexane;water; for 24 h;
Steps:
b.5 4.2 Typical procedure for the synthesis of 1a
General procedure: To a solution of 2-methylpyridine (2.79 g, 30 mmol) in 35 mL THF, nBuLi (12.4 mL, 2.5 M solution in hexane, 30 mmol) was slowly added at 0 °C with stirring. The mixture was allowed to warm to room temperature for 4 h, after which acetonitrile (1.61 mL, 31 mmol) was added at 0 °C and then stirred for 6 h at room temperature. A sulfuric acid of 60% aqueous solution was then added dropwise until a PH of 1 was reached, and was stirred for 1 d to complete acidic hydrolysis, then neutralized with saturated aqueous KOH solution. The organic layer was extracted with CH2Cl2 (50 mL × 3), then dried over MgSO4 and concentrated by rotary evaporator. This compound was purified by reduced pressure distillation, resulting in yellow oil of 1a. 1b-2g were purified by reduced pressure distillation. 3a-3f were purified by recrystallization and chromatography.
References:
Bai, Jianliang;Wang, Peng;Cao, Wei;Chen, Xia [Journal of Molecular Structure,2017,vol. 1128,p. 645 - 652]
109-06-8
420 suppliers
$16.00/25g

72076-59-6
13 suppliers
inquiry