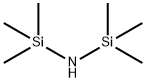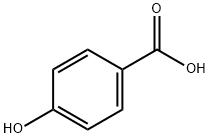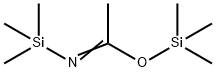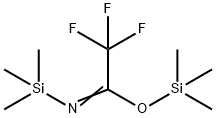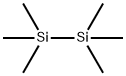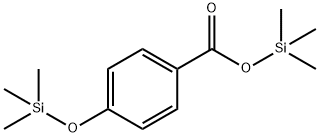
4-[(TRIMETHYLSILYL)OXY]-BENZOIC ACID TRIMETHYLSILYL ESTER synthesis
- Product Name:4-[(TRIMETHYLSILYL)OXY]-BENZOIC ACID TRIMETHYLSILYL ESTER
- CAS Number:2078-13-9
- Molecular formula:C13H22O3Si2
- Molecular Weight:282.48
Yield:2078-13-9 94%
Reaction Conditions:
with iodine in neat (no solvent) at 20; for 0.67 h;Green chemistry;
Steps:
34 Representative procedure of I2-catalyzed functionalization of carboxylic acids with HMDS
General procedure: To a mixture of decanoic acid 1b (1.72 g, 10 mmol) and HMDS (1.61 g, 10 mmol), iodine (0.15 g, 0.6 mmol) was added, and the mixture was stirred at room temperature until the complete conversion of 1b. The initial heterogeneous reaction mixture became homogeneous, and 1H NMR analysis confirmed full conversion. A brown reaction mixture was diluted with a few drops of TBME, and anhydrous sodium thiosulfate was added in small portions during stirring at room temperature. After some time of stirring, the reaction mixture decolorized (the color of iodine disappeared). In some cases, the color of iodine persisted for hours, and then small portions of sodium thiosulfate pentahydrate were added. Further stirring reduced iodine, and the brown color disappeared. In a few cases, iodine disappeared during the reaction, and its removal was not needed. The reaction mixture was diluted with a small volume of TBME and filtered. The filtrate was concentrated under reduced pressure, and the thus-obtained reaction mixture was subjected to a vacuum distillation. After removal of excess of HMDS, the distillation of crude product gave trimethylsilyl decanoate 2b (1.18 g, 48%) as a slightly yellow oil. The distilled excess of HMDS could be reused.
References:
Jereb, Marjan;Lakner, Janja [Tetrahedron,2016,vol. 72,# 37,p. 5713 - 5723]