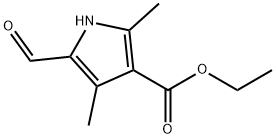
Ethyl 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylate synthesis
- Product Name:Ethyl 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylate
- CAS Number:2199-59-9
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
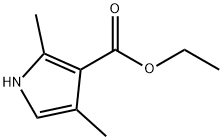
2199-51-1

2199-59-9
The general procedure for the synthesis of ethyl 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylate from ethyl 2,4-dimethylpyrrole-3-carboxylate was as follows: dimethylformamide (322 g) was mixed with dichloromethane (3700 mL) and cooled to 4 °C in an ice bath, followed by the slow addition of phosphorus trichloride (684 g) under stirring. Solid ethyl 2,4-dimethyl-1H-pyrrole-3-carboxylate (670 g) was added in batches over 15 min and the maximum temperature was controlled at 18 °C during the reaction. The reaction mixture was heated to a reflux state maintained for 1 h. Subsequently, the temperature was raised to 15 °C by cooling to 10 °C and rapid addition of 1.6 L of ice water. 10N hydrochloric acid (1.6L) was added with vigorous stirring and the temperature was raised to 22°C. The reaction was allowed to stand for 30 minutes to allow the two phases to separate. The two phases were allowed to separate by standing for 30 minutes, during which time the maximum temperature reached 40°C. The pH of the aqueous phase was adjusted to 12-13 with 10N potassium hydroxide (3.8L), and the temperature was maintained at 55°C during the adjustment. After adjustment, the mixture was cooled to 10°C and stirred for 1 hour. The product was collected by vacuum filtration and washed four times with water to afford ethyl 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylate (778 g, 100% yield) as a yellow solid. The product was analyzed by 1H-NMR (DMSO-d6) and mass spectrometry to confirm the structure: 1H-NMR δ 1.25 (t, 3H, CH3), 2.44,2.48 (2*s, 2*3H, 2*CH3), 4.16 (q, 2H, CH2), 9.59 (s, 1H, CHO), 12.15 (br s, 1H, NH); MS m/z 195 [M+1].

2199-51-1
242 suppliers
$7.00/250mg

2199-59-9
232 suppliers
$8.00/250mg
Yield:2199-59-9 100%
Reaction Conditions:
with hydrogenchloride in N-methyl-acetamide;(2S)-N-methyl-1-phenylpropan-2-amine hydrate;dichloromethane;trichlorophosphate;
Steps:
80 5-[5-Fluoro-2-oxo-1,2-dihydro-indol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic Acid (2-diethylamino-ethyl)-amide
Dimethylformamide (322 g) and dichloromethane (3700 mL) were cooled in an ice bath to 4° C. and phosphorus oxychloride (684 g) was added with stirring. Solid 2,4-dimethyl-1H-pyrrole-3-carboxylic acid ethyl ester (670 g) was slowly added in aliquots over 15 minutes. The maximum temperature reached was 18° C. The mixture was heated to reflux for one hour, cooled to 10° C. in an ice bath and 1.6 L of ice water was rapidly added with vigorous stirring. The temperature increased to 15° C. 10 N Hydrochloric acid (1.6 L) was added with vigorous stirring. The temperature increased to 22° C. The mixture was allowed to stand for 30 minutes and the layers allowed to separate. The temperature reached a maximum of 40° C. The aqueous layer was adjusted to pH 12-13 with 10 N potassium hydroxide (3.8 L) at a rate that allowed the temperature to reach and remain at 55° C. during the addition. After the addition was complete the mixture was cooled to 10° C. and stirred for 1 hour. The solid was collected by vacuum filtration and washed four times with water to give 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic acid ethyl ester (778 g, 100% yield) as a yellow solid. 1H-NMR (DMSO-d6) δ 1.25 (t, 3H, CH3), 2.44, 2.48 (2*s, 2*3H, 2*CH3), 4.16 (q, 2H, CH2), 9.59 (s, 1H, CHO), 12.15 (br s, 1H, NH). MS m/z 195 [M+1].
References:
US2003/216410,2003,A1
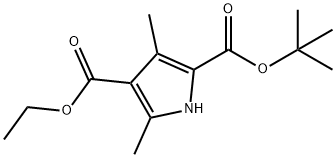
86770-31-2
154 suppliers
$19.78/1gm:

2199-59-9
232 suppliers
$8.00/250mg

64-17-5
827 suppliers
$10.00/50g
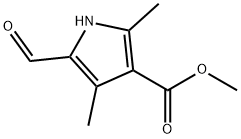
58298-68-3
21 suppliers
$255.00/1g

2199-59-9
232 suppliers
$8.00/250mg

56453-92-0
0 suppliers
inquiry

2199-59-9
232 suppliers
$8.00/250mg

141-97-9
822 suppliers
$5.00/25g

2199-59-9
232 suppliers
$8.00/250mg