(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester synthesis
- Product Name:(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester
- CAS Number:229613-93-8
- Molecular formula:C18H30N2O5
- Molecular Weight:354.44
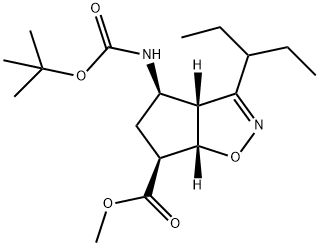
![(3aR,4R,6S,6aS)-4-(tert-butoxycarbonylaMino)-3-(pentan-3-yl)-4,5,6,6a-tetrahydro-3aH-cyclopenta[d]isoxazole-6-carboxylic acid](/CAS/GIF/316173-28-1.gif)
316173-28-1

77-78-1
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
1. (3aR,4R,6S,6aS)-4-((tert-butoxycarbonyl)amino)-3-(pentan-3-yl)-3a,5,6,6a-tetrahydro-4H-cyclopenta[d]isoxazole-6-carboxylic acid 2-methylpropan-2-ammonium salt (3210 g, 7.8 mol) was suspended in acetone (4000 g). 2. potassium carbonate (53.8 g) and 30% aqueous sodium hydroxide (containing 312 g sodium hydroxide) were added to the suspension. 3. 3000 ml of solvent was removed by evaporation, 3000 ml of acetone was added and 3000 ml of solvent was again removed by evaporation. This operation was repeated once, and finally 4000 ml of acetone was removed by evaporation to obtain a reaction solution essentially free of tert-butylamine odor. 4. Cool the reaction solution to 30-35°C and slowly add 1060 ml of dimethyl sulfate dropwise, controlling the rate of dropwise acceleration in order to keep the reaction temperature from exceeding 45°C. After the dropwise acceleration is completed, the reaction solution is added to the reaction solution. 5. After the dropwise addition, the reaction mixture was stirred at 40-45°C for 1.5 hours. 6. The reaction mixture was cooled to 15-20 °C, 1500 ml of 25% ammonia was added and stirred for 30 minutes. 7. After addition of 1500 ml of methanol, the reaction mixture was cooled to 0-5 °C. 8. 2240 ml of 25% ammonia was slowly added to the reaction mixture over 45 minutes. 9. The product was collected by filtration, washed with 3000 ml of water and dried under vacuum at 45-50 °C. 10. 2686.2 g of the target product was finally obtained in 97.13% yield.
![(3aR,4R,6S,6aS)-4-(tert-butoxycarbonylaMino)-3-(pentan-3-yl)-4,5,6,6a-tetrahydro-3aH-cyclopenta[d]isoxazole-6-carboxylic acid](/CAS/GIF/316173-28-1.gif)
316173-28-1
53 suppliers
$355.00/100mg

77-78-1
320 suppliers
$22.00/25g
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
204 suppliers
$10.00/1g
Yield:229613-93-8 97.13%
Reaction Conditions:
Stage #1: (3aR,4R,6S,6aS)-4-[[(1,1-dimethylethoxy)carbonyl]amino]-3-(1'-ethylpropyl)-3a,5,6,6a-tetrahydro-4H-cyclopent[d]isoxazole-6-carboxylic acid tert-butylaminewith potassium carbonate;sodium hydroxide in water;acetone;Industry scale;
Stage #2: dimethyl sulfate in water;acetone at 30 - 45;
Stage #3: with ammonia in methanol;water;acetone at 0 - 20; for 1.25 h;Industry scale;
Steps:
3 Synthesis of methyl (3aR,4R,6S,6aS)-4-[[(1,1-dimethylethoxy)carbonyl]amino]-3-(1'-ethylpropyl)-3a,5,6,6a-tetrahydro-4H-cyclopenta[d]isooxazole-6-carboxylate (5)
1,1-dimethylethyl ammonium (3aR,4R,6S,6aS)-4-[[(1,1-dimethylethoxy) carbonyl] amino]-3-(1'-ethylpropyl)-3a,5,6,6a-tetrahydro-4H-cyclopenta[d]isooxazole-6-carboxylate (16) (3210 g, 7.8 mol) was suspended in acetone (4000 g). Potassium carbonate (53.8 g), and an aqueous solution of 30% sodium hydroxide containing 312 g of sodium hydroxide were added to the resulting suspension. 3000 ml of solvent was removed by evaporation, 3000 ml of acetone was added, then, 3000 ml of solvent was removed by evaporation, 3000 ml of acetone was added, and thereafter 4000 ml acetone was removed by evaporation, obtaining a reaction solution substantially free of the odor of tert-butylamine. The reaction solution was cooled down to 30 - 35°C, to which 1060 ml of dimethyl sulfate was added dropwise at a speed to keep the temperature not exceeding 45°C. After completion of the addition, the resulting reaction mixture was stirred at 40 - 45°C for 1.5 hours. The reaction mixture was cooled down to 15 - 20°C, to which was then added 1500 ml of 25% ammonia. After stirring for 30 minutes, 1500 ml of methanol was added, and then the reaction mixture was cooled down to 0 - 5°C. Thereafter, 2240 ml of 25% ammonia was added to the reaction mixture within 45 minutes. The resulting product was collected by filtration, washed with 3000 ml of water, and dried under vacuum at 45 - 50°C. Finally, 2686.2 g of a product was obtained, with a yield of 97.13%.
References:
EP2186795,2010,A1 Location in patent:Page/Page column 6; 11-12
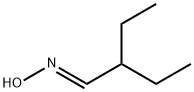
49805-57-4
0 suppliers
inquiry
![4-[[(1,1-Dimethylethoxy)carbonyl]amino]-2-cyclopentene-1-carboxylic acid methyl ester](/CAS/GIF/168683-02-1.gif)
168683-02-1
155 suppliers
$161.00/10g
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
204 suppliers
$10.00/1g
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
220 suppliers
$10.00/1g
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
204 suppliers
$10.00/1g
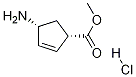
229613-83-6
43 suppliers
inquiry
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
204 suppliers
$10.00/1g
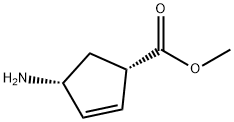
138923-03-2
32 suppliers
$333.00/250mg
![(1S-4R)-4-[[(1,1-diMethylethoxy)carbonyl]aMino]- 2-Cyclopentene-1-carboxylic acid Methyl ester](/CAS/20150408/GIF/229613-93-8.gif)
229613-93-8
204 suppliers
$10.00/1g