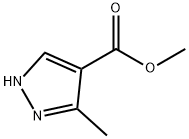
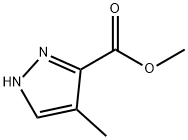
5-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:5-METHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID METHYL ESTER
- CAS Number:23170-45-8
- Molecular formula:C6H8N2O2
- Molecular Weight:140.14

67-56-1
40704-11-8

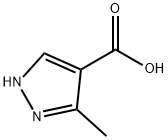
23170-45-8
General procedure for the synthesis of ethyl 3-methyl-1H-pyrazole-4-carboxylate from methanol and 3-methylpyrazole-4-carboxylic acid: 3-methylpyrazole-4-carboxylic acid (1.26 g, 10 mmol) was dissolved in methanol (100 mL) at 0 °C and thionyl chloride (5.73 g, 45 mmol) was added slowly, the reaction mixture was stirred at room temperature for 12 hours. After completion of the reaction, the solvent was removed by vacuum evaporation. The residue was neutralized by adding saturated aqueous sodium bicarbonate solution and the mixture was extracted with ethyl acetate (100 mL x 3). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and the filtrate was concentrated in vacuum to give ethyl 3-methyl-1H-pyrazole-4-carboxylate (0.8 g, 57% yield). The product was characterized by 1H NMR (300 MHz, CDCl3): δ 7.87 (s, 1H), 3.84 (s, 3H), 2.53 (s, 3H).

67-56-1
790 suppliers
$7.29/5ml-f

40704-11-8
113 suppliers
$9.00/250mg

23170-45-8
72 suppliers
$53.00/100mg
Yield: 57%
Reaction Conditions:
with thionyl chloride at 0; for 12 h;
Steps:
5 Synthesis of methyl 3-rnetIiy1-1H-pyraioie-4-carboxyIate.
Synthesis of /V-((2-hydroxy-4,( methyl-l-(l-benzrl)-l /-pyrazoie-4-carboxamide (Compound 11 esis 14] To a solution of 3-methyi-lH-pyrazole-4-carboxylic acid (1.26 g, 10 mmol) in methanol (100 mL) was added thionyl chloride (5.73 g, 45 mmol) at 0 °C. The mixture was stirred for 12 hours. The solvent was evaporated in vacuo. To the residue, saturated sodium bicarbonate aqueous solution was added and the mixture was extracted with ethyl acetate (100 mLx 3), the organic phase was dried by sodium, sulfate. The mixture was filtered and the filtrate was concentrated in vacuo to give methyl 3-m.ethyl-lH~pyrazole~4~carboxylate (0.8 g, 57%). H NMR (300 MHz, CDCI3): δ 7.87 (s, 1H), 3.84 (s, 3H), 2.53 (s, 3H).
References:
CONSTELLATION PHARMACEUTICALS;ALBRECHT, Brian, K.;AUDIA, James, Edmund;COOK, Andrew, S.;DAKIN, Les, A.;DUPLESSIS, Martin;GEHLING, Victor, S.;HARMANGE, Jean-Christophe;NAVESCHUK, Christopher, G.;VASWANI, Rishi, G. WO2013/120104, 2013, A2 Location in patent:Paragraph 00183; 00184
4637-24-5
646 suppliers
$5.00/25g
105-45-3
694 suppliers
$5.00/5g

23170-45-8
72 suppliers
$53.00/100mg
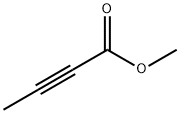
23326-27-4
145 suppliers
$38.13/1g

18107-18-1
211 suppliers
$33.00/5g
68809-58-5
49 suppliers
$144.00/50mg

23170-45-8
72 suppliers
$53.00/100mg

105-45-3
694 suppliers
$5.00/5g

23170-45-8
72 suppliers
$53.00/100mg