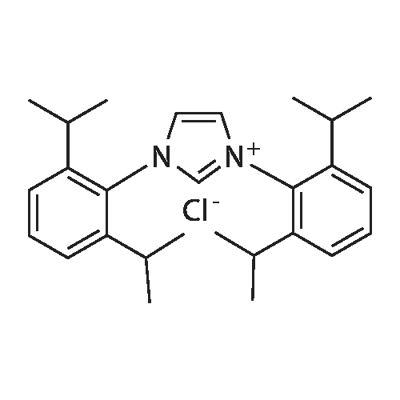
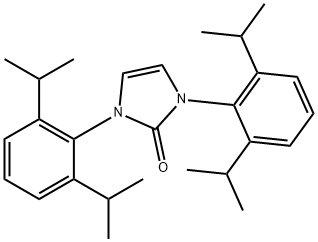
1,3-Bis (2,6-diisopropylphenyl) imidazolium chloride synthesis
- Product Name: 1,3-Bis (2,6-diisopropylphenyl) imidazolium chloride
- CAS Number:250285-32-6
- Molecular formula:C27H37ClN2
- Molecular Weight:425.05

50-00-0
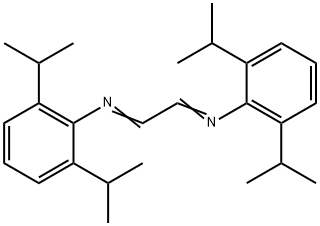
74663-75-5

250285-32-6
1. Methanol (500 mL), 2,6-diisopropylaniline (63.8 mL, 340 mmol), glyoxal (40 wt% aqueous solution, 19 mL, 170 mmol), and formic acid (1 mL) were added to a 1000 mL round bottom flask. The reaction mixture was stirred at room temperature for 3 hours. 2. The reaction mixture was filtered and the yellow precipitate (3) was collected and washed with cold methanol. The precipitate was dried under vacuum overnight to give product (3) (70% yield, 44.2 g, 238 mmol). 3. The product (3) (200 g, 532 mmol) and ethyl acetate (2 L) were added to a 5 L round bottom flask and stirred until completely dissolved. Cool the solution to 0°C. 4. To a 500mL conical flask was added paraformaldehyde (20.7g, 690mmol) and a dioxane solution of 4N HCl (212mL, 851mmol), stirred for 10 minutes and then added to the above cooled solution. 5. The reaction mixture was stirred at room temperature for 2 hours and the precipitate was collected by filtration. 6. The precipitate was dissolved in methanol (200 mL), sodium bicarbonate (15.0 g) was added and stirred for 1 hour or until no bubbles were produced. 7. The solids were removed by filtration and the filtrate was reprecipitated by adding diethyl ether (250 mL) to the filtrate. 8. The product was collected by filtration, washed with ethyl ether and dried under vacuum to afford 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride (IPr-HCl, 4) as a white powder (70% yield, 158.25 g, 371 mmol). 9. The product was characterized by 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 400 MHz) and the data were consistent with the expected structure.

50-00-0
896 suppliers
$10.00/25g

74663-75-5
52 suppliers
$172.00/1g

250285-32-6
257 suppliers
$6.00/250mg
Yield:250285-32-6 89%
Reaction Conditions:
with chloro-trimethyl-silane in ethyl acetate at 70; for 2 h;
Steps:
4.3. Preparation of the catalyst
i. A mixture of 2,6-diisopropylaniline (33.9 mmol) and HOAc (1.18 mmol) and 15 mL MeOH was stirred in at 50 °C, then slowly dropwise added 15 mL of a MeOH solution of glyoxal (40% aqueous solution, 16.7 mmol) in 15 minutes. After the addition, the mixture continuing to stir at 50 °C for 30 minutes and then at room temperature for 10 hours. The reaction mixture was filtered dried to obtain 5.6 g of yellow compound a[57] (87% yield). ii. a (8.5 mmol) and paraformaldehyde (8.5 mmol) were added into 30 mL EtOAc and stirred vigorously to dissolve at 70 °C. Then, 20 mL of TMSCl (0.85 mmol) in EtOAc was slowly added dropwise to the reaction flask within 20 minutes. After reacting for 2 h, the reaction mixture is cooled to 10 °C and filtered. The filter cake was washed with EtOAc and dried to obtain 3.2 g of compound b[57] as a white solid (89% yield).
References:
Li, Dan;Tian, Qingqiang;Wang, Xuetong;Wang, Qiang;Wang, Yin;Liao, Siwei;Xu, Ping;Huang, Xin;Yuan, Jianyong [Synthetic Communications,2021,vol. 51,# 13,p. 2041 - 2052] Location in patent:supporting information

74663-75-5
52 suppliers
$172.00/1g
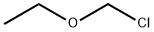
3188-13-4
169 suppliers
$35.00/5G

250285-32-6
257 suppliers
$6.00/250mg

50-00-0
896 suppliers
$10.00/25g

74663-75-5
52 suppliers
$172.00/1g

250285-32-6
257 suppliers
$6.00/250mg
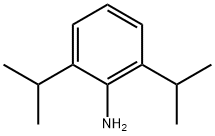
24544-04-5
352 suppliers
$5.00/10g

250285-32-6
257 suppliers
$6.00/250mg
![CHLORO[1,3-BIS(2,6-DI-I-PROPYLPHENYL)IMIDAZOL-2-YLIDENE]COPPER(I)](/CAS/GIF/578743-87-0.gif)
578743-87-0
99 suppliers
$29.00/250mg
1060651-05-9
12 suppliers
inquiry

250285-32-6
257 suppliers
$6.00/250mg