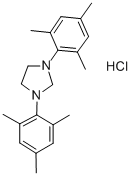
1,3-BIS(2,4,6-TRIMETHYLPHENYL)-IMIDAZOLIDINIUM-CHLORIDE synthesis
- Product Name:1,3-BIS(2,4,6-TRIMETHYLPHENYL)-IMIDAZOLIDINIUM-CHLORIDE
- CAS Number:173035-10-4
- Molecular formula:C21H29ClN2
- Molecular Weight:344.92
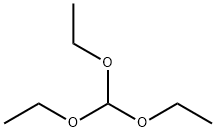
122-51-0
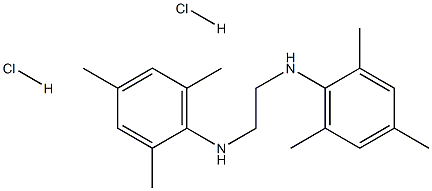
258278-23-8

173035-10-4
Sodium borohydride (NaBH4, 4.24 g, 112.1 mmol) was slowly added to a solution of N,N'-bis(2,4,6-trimethylphenyl)ethylenediamine hydrochloride (8.0 g, 27.3 mmol) in tetrahydrofuran (THF, 100 mL) at 0 °C. Subsequently, concentrated hydrochloric acid (HCl, 4.5 mL, 2 eq.) was added dropwise over 30 min. After the dropwise addition, the reaction mixture was continued to be stirred at 0 °C for 20 min. Next, 3M hydrochloric acid (250 mL) was carefully added to the reaction flask at 0°C and the mixture was stirred for 1 hour, during which time the temperature was allowed to naturally rise to room temperature. Upon completion of the reaction, the white precipitate was collected by filtration and washed sequentially with water (200 mL) and 5% acetone-ether mixture (150 mL). The resulting product (9.4 g, 93% yield) was a white solid, which was dried under vacuum and prepared for use. The above obtained hydrochloride (8.5 g, 23 mmol) was suspended in triethyl orthoformate (HC(OEt)3, 35 mL, 162 mmol) and 2 drops of formic acid (HCO2H, ca. 1 mol%) was added as catalyst. The reaction mixture was heated to 120 °C and maintained for 5 hours under argon (Ar) protection. At the end of the reaction, it was cooled to room temperature and hexane (200 mL) was added to precipitate the product. After stirring for 1 hour, the white precipitate was collected by filtration, washed with hexane (200 mL) and dried under vacuum to give the final 1,3-bis(2,4,6-trimethylphenyl)imidazole hydrochloride (IMesH2-HCl, 7.6 g, 96% yield).

64-18-6
1132 suppliers
$22.12/250ML

258278-23-8
10 suppliers
inquiry

173035-10-4
188 suppliers
$43.00/500mg
Yield:173035-10-4 80%
Reaction Conditions:
with orthoformic acid triethyl ester at 23 - 130;Inert atmosphere;
Steps:
1,3-Bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazolium chloride5
A mixture of 1.12 g (3.03 mmol) of N,N'-bis-(2,4,6-trimethylphenylamino)ethane dihydrochloride, 10mL of triethyl orthoformate, and one drop of 96% formic acid was heated in a distillation apparatus until the ethanol distillation ceased. The temperature of the reaction mixture reached 130 oC. Uponcooling to 23 oC, a colorless solid precipitated which was collected by filtration and dried in vacuo.
References:
Zeng, Wei;Wang, Enyu;Qiu, Rui;Sohail, Muhammad;Wu, Shaoxiang;Chen, Fu-Xue [Journal of Organometallic Chemistry,2013,vol. 743,p. 44 - 48] Location in patent:supporting information

122-51-0
514 suppliers
$10.00/5ml

258278-23-8
10 suppliers
inquiry

173035-10-4
188 suppliers
$43.00/500mg

122-51-0
514 suppliers
$10.00/5ml
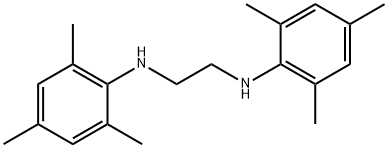
134030-21-0
85 suppliers
$45.00/50mg

173035-10-4
188 suppliers
$43.00/500mg
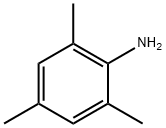
88-05-1
351 suppliers
$6.00/1g

173035-10-4
188 suppliers
$43.00/500mg
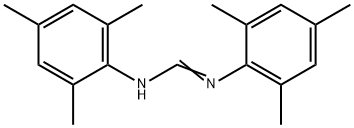
75105-48-5
2 suppliers
inquiry

107-06-2
441 suppliers
$14.00/25g

173035-10-4
188 suppliers
$43.00/500mg