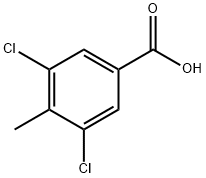
3,5-Dichloro-4-methylbenzoic acid synthesis
- Product Name:3,5-Dichloro-4-methylbenzoic acid
- CAS Number:39652-34-1
- Molecular formula:C8H6Cl2O2
- Molecular Weight:205.04
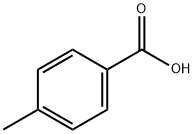
99-94-5

39652-34-1
To a 1000 mL four-neck reaction flask (equipped with a mechanical stirrer, thermometer, dropping funnel, and reflux condenser) was added 136 g of p-toluic acid followed by 272 g of dichloroacetic acid as a solvent. Next, 0.2 g of sodium tungstate dihydrate was added as catalyst and 365 g of 30% hydrochloric acid was added. Stirring was initiated and the reaction mixture was heated to 50-60 °C. At this temperature, 136 g of 30% hydrogen peroxide was slowly added dropwise, and the dropwise process was controlled to be completed within 6 hours, during which the temperature was maintained at 50-60 °C. After the dropwise addition, the reaction was continued for 4 hours. The reaction mixture was analyzed by high performance liquid chromatography (HPLC) and the remaining content of p-toluic acid was confirmed to be 9.5%. After completion of the reaction, the reaction solution was distilled under reduced pressure. When no fraction evaporated, the temperature was lowered to 20-30 °C, 250 g of methanol was added, heated to reflux and then cooled to 10 °C again. The reaction mixture was filtered, the solid residue was collected and the filter cake was washed with methanol. The filter cake was dried to give a final product of 165.2 g of 3,5-dichloro-4-methylbenzoic acid in 80.6% yield and 98.5% HPLC purity.

99-94-5
611 suppliers
$9.00/1g

39652-34-1
101 suppliers
$8.00/100mg
Yield: 81%
Reaction Conditions:
aluminium trichloride in dichloromethane
Steps:
20.a a)
a) Preparation of 3,5-dichloro-4-methylbenzoic acid To a solution of p-toluic acid (95.0 g, 0.698 mole) in methylene chloride (1 liter), was added aluminum chloride (260.0 g, 1.948 mole) portionwise keeping the reaction temperature below 10° C. (ice-water bath). When the addition was completed (approx. 30 minutes) chlorine gas was bubbled in at such a rate as to keep the temperature below 10° C. The reaction was followed by gas-liquid chromatography. After about 4 hours, most of the starting material had been converted to the expected compound. The resulting mixture was poured into ice and concentrated hydrochloric acid and then extracted with ethyl acetate several times. The combined organic layers were then washed with water and dried over anhydrous sodium sulfate. Removing the solvent in a rotavap yielded the crude product as a white solid. Recrystallization from acetone/water or ethyl acetate/hexane yielded 3,5-dichloro-4-methylbenzoic acid with minor impurities 115.4 g (81% yield of product).
References:
Rohm and Haas Company US5254584, 1993, A

99-94-5
611 suppliers
$9.00/1g
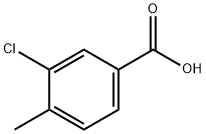
5162-82-3
167 suppliers
$10.00/1g

39652-34-1
101 suppliers
$8.00/100mg

124-38-9
134 suppliers
$175.00/23402
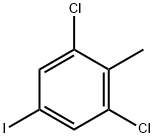
177167-52-1
6 suppliers
inquiry

39652-34-1
101 suppliers
$8.00/100mg

124-38-9
134 suppliers
$175.00/23402
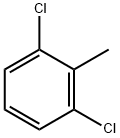
118-69-4
295 suppliers
$14.00/5g
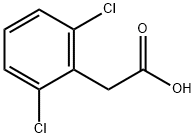
6575-24-2
298 suppliers
$8.00/5g

39652-34-1
101 suppliers
$8.00/100mg

83277-23-0
89 suppliers
$37.00/100mg
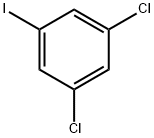
3032-81-3
221 suppliers
$10.00/1g

39652-34-1
101 suppliers
$8.00/100mg