3,5-Difluorochlorobenzene synthesis
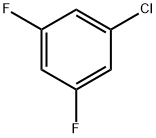
- Product Name:3,5-Difluorochlorobenzene
- CAS Number:1435-43-4
- Molecular formula:C6H3ClF2
- Molecular Weight:148.54
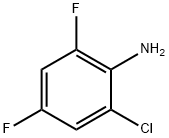
36556-56-6

1435-43-4
General procedure for the synthesis of 3,5-difluorochlorobenzene from 6-chloro-2,4-difluoroaniline: 1. 0.5 moles (1.0 equiv.) of 6-chloro-2,4-difluoroaniline and 200 ml of dichloromethane were added to a reaction flask and stirred until completely dissolved. 2. 0.55 moles (1.1 eq.) of N-chlorosuccinimide (NCS) was added in 5 batches, the reaction temperature was controlled at 10~15°C, and after the addition was completed, the reaction was heated to reflux for 2 hours. 3. The completion of the reaction was monitored by TLC and the pH was adjusted to 6-7 by adding 10% dilute hydrochloric acid to the reaction mixture. 4. The organic phase was separated, washed with water and rotary evaporated to dryness to give 0.44 mol (0.88 eq.) of 2,4-difluoro-5-chloroaniline. 5. 0.44 mol of 2,4-difluoro-5-chloroaniline was added to 1.5 mol (3 eq.) of 25% dilute sulfuric acid and stirred for 0.5 hr. 6. Add 0.75 mol (1.5 eq.) of sodium phosphite, control the reaction temperature at 30~35°C, slowly add 0.5 mol (1 eq.) of aqueous sodium nitrite dropwise, control the rate of dropwise acceleration so that the reaction temperature rises 4~5°C per hour. 7. Warm up to 60°C, keep the temperature stirring for 2 hours. 8. 8. leave to stratify, the organic phase is washed with water and then steam distilled. 9. 9. 0.41 moles (0.82 equivalents) of 3,5-difluorochlorobenzene were obtained by distillation, with a purity of 99.8%.
108-70-3
256 suppliers
$19.89/442235
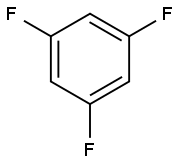
372-38-3
324 suppliers
$9.00/1g

1435-43-4
221 suppliers
$7.00/5g
Yield:372-38-3 74% ,1435-43-4 6.7%
Reaction Conditions:
with potassium fluoride;3-nitro-N,N-dimethylbenzamide;1,3-dimethyl-2-(N',N',N",N"-tetramethylguanidino)-4,5-dihydro-3H-imidazolium chloride in sulfolane at 220; under 6000.6 Torr; for 48 h;
Steps:
4
Example 4; Preparation method of 1,3,5-trifluorobenzene; 500 g of 1,3,5-trichlorobenzene, 1180 ml of sulpholane, 10.7 g of 3-nitrodimethylbenzamide and 640 g of dry KF were initially charged in an autoclave, then 48 g of CNC catalyst were added and the autoclave was sealed. The mixture was heated to 220° C. for 48 h. During the reaction, a maximum total pressure of 8 bar arose. Subsequently, the mixture was cooled to 20° C. The product was distilled off initially at standard pressure, later under reduced pressure. 310 g of a colourless liquid having a proportion of 87% by weight of 1,3,5-trifluorobenzene (74% of theory) and 8.8% by weight of difluorochlorobenzene (6.7% by weight of theory) were obtained. 1,3,5-Trifluorobenzene and difluorochlorobenzene can be separated distillatively in a known manner.
References:
Pleschke, Axel;Marhold, Albrecht US2006/9643, 2006, A1 Location in patent:Page/Page column 5

108-70-3
256 suppliers
$19.89/442235

1435-43-4
221 suppliers
$7.00/5g

36556-56-6
161 suppliers
$7.00/1g

1435-43-4
221 suppliers
$7.00/5g
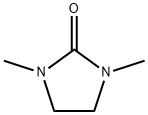
80-73-9
598 suppliers
$8.00/25g

108-70-3
256 suppliers
$19.89/442235
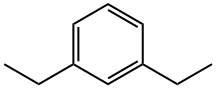
141-93-5
135 suppliers
$44.00/2ml

1435-43-4
221 suppliers
$7.00/5g
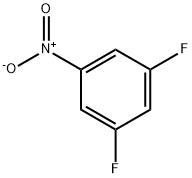
2265-94-3
230 suppliers
$10.00/1g

1435-43-4
221 suppliers
$7.00/5g