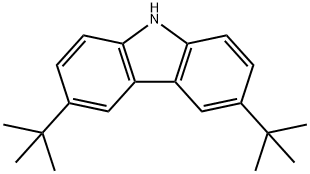
3,6-Di-tert-butylcarbazole synthesis
- Product Name:3,6-Di-tert-butylcarbazole
- CAS Number:37500-95-1
- Molecular formula:C20H25N
- Molecular Weight:279.42
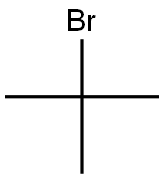
507-20-0
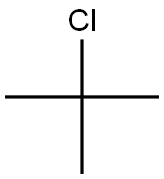
86-74-8

37500-95-1
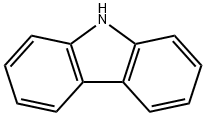
9H-carbazole (1) (5.00 g, 29.9 mmol) was dissolved in dichloromethane and tert-butyl chloride (6.6 ml, 59.8 mmol) and anhydrous aluminum chloride (4.00 g, 29.9 mmol) were added sequentially. The reaction mixture was stirred at 25°C for 24 hours. Upon completion of the reaction, the mixture was poured into ice water (100 mL) to quench the reaction. The organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate and filtered to remove the desiccant. The filtrate was concentrated under reduced pressure at room temperature to give the crude product. The crude product was recrystallized by petroleum ether to give 6.30 g of 3,6-di-tert-butyl-9H-carbazole (2a) as a white solid in 75% yield.

507-20-0
384 suppliers
$13.00/25mL

86-74-8
347 suppliers
$10.00/5g
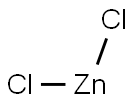
7646-85-7
605 suppliers
$10.00/25ml

37500-95-1
242 suppliers
$6.00/1g
Yield:37500-95-1 45%
Reaction Conditions:
in nitromethane;water
Steps:
P.1 Synthesis of -3,6-di-tert-butylcarbazole (YZ-I-1)
Preparative Example 1 Synthesis of -3,6-di-tert-butylcarbazole (YZ-I-1) To a solution of carbazole (6.6 g, 39.5 mmol) and zinc (II) chloride (16.2 g, 118.8 mmol) in nitromethane (100.0 ml) was added dropwise 2-chloro-2-methylpropane (11.1 g, 120.0 mmol) under nitrogen atmosphere and stirring. The addition of 2-chloro-2-methylpropane was carried out over a 15 minute period. The mixture was stirred at room temperature for 24 hours. Water (100.0 ml) was then added. The product was extracted with dichloromethane (3*60.0 ml). The organic layer was washed with water (3*100.0 ml), and then dried with MgSO4, and dichloromethane was evaporated under vacuum. The product was purified by recrystallization from hot hexane to afford a white product crystal in the amount of 5.0 g (in 45.0% yield). 1H NMR (CDCl3): δ 8.06 (d, 2HCz, J=1.6 Hz), 7.83 (s, br, 1H, NH), 7.45 (dd, 2HCz, J1=8.8 Hz, J2=1.6 Hz), 7.30 (d, 2HCz, J=8.8 Hz), 1.45 (s, 18H, 9*CH3). 13C NMR (CDCl3): δ 142.04, 137.84, 123.38, 123.18, 116.06, 109.88, 24.78, 32.13.
References:
GEORGIA TECH RESEARCH CORPORATION US2010/331509, 2010, A1

507-20-0
384 suppliers
$13.00/25mL

86-74-8
347 suppliers
$10.00/5g

37500-95-1
242 suppliers
$6.00/1g
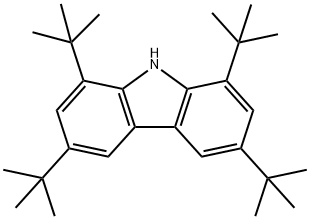
34601-54-2
32 suppliers
$105.00/200mg

37500-95-1
242 suppliers
$6.00/1g

507-20-0
384 suppliers
$13.00/25mL

86-74-8
347 suppliers
$10.00/5g

34601-54-2
32 suppliers
$105.00/200mg
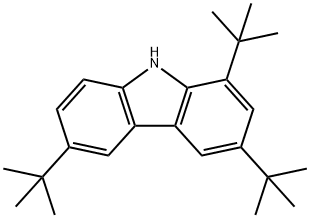
34601-53-1
0 suppliers
inquiry

37500-95-1
242 suppliers
$6.00/1g