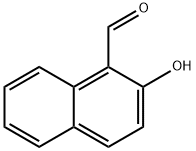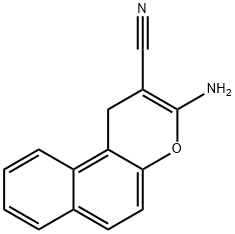
3-Amino-1H-benzo[f]chromene-2-carbonitrile synthesis
- Product Name:3-Amino-1H-benzo[f]chromene-2-carbonitrile
- CAS Number:141987-72-6
- Molecular formula:C14H10N2O
- Molecular Weight:222.24
![3-imino-3H-benzo[f]chromene-2-carbonitrile](/CAS/20180808/GIF/80860-05-5.gif)
80860-05-5
3 suppliers
inquiry
![3-Amino-1H-benzo[f]chromene-2-carbonitrile](/CAS/20140525/GIF/CB32555561.gif)
141987-72-6
6 suppliers
inquiry
Yield:141987-72-6 90%
Reaction Conditions:
with sodium tetrahydroborate in methanol at 0; for 0.75 h;
Steps:
General procedure for the synthesis of 2-amino-4H-1-chromene-3-carbonitrile 4(a-f) by reduction of 3-cyano2-iminocoumarins 3(a-f).
General procedure: In a 50 mL round-bottomed flask, provided with a magnetic stirrer and condenser,containing a suspension of 2-imino-2H-1-benzopyran-3-carbonitrile 3 (12 mmol.) in 12 mL of methanol cooledat 0°C was added small portions of commercial sodium borohydride (227 mg, 6 mmol.). The resulting reactionmixture was stirred vigorously (500 rpm) at 0 °C during 45 min. After stirring, the mixture was poured indeionized water at room temperature without stirring to improve decantation and precipitation. The resultingprecipitate was collected by filtration on a Büchner funnel (porosity N°4) and washed with deionized water (3x 10 mL) . The desired 2-amino-4H[1]chromene-3-carbonitrile 4 was dried under high vacuum (10-2 Torr) at25°C for 1 h that gave a yellowish powder and was further used without purification.
References:
Bouattour, Ali;Fakhfakh, Mehdi;Abid, Souhir;Paquin, Ludovic;Le Guével, Rémy;Corlu, Anne;Ruchaud, Sandrine;Bach, Stéphane;Ammar, Houcine;Bazureau, Jean-Pierre [Arkivoc,2017,vol. 2017,# 4,p. 291 - 302]
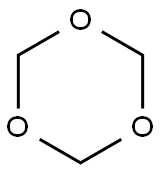
110-88-3
259 suppliers
$13.00/100g
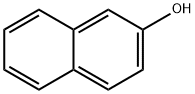
135-19-3
754 suppliers
$9.00/1g

109-77-3
452 suppliers
$10.00/5g
![3-Amino-1H-benzo[f]chromene-2-carbonitrile](/CAS/20140525/GIF/CB32555561.gif)
141987-72-6
6 suppliers
inquiry

50-00-0
897 suppliers
$10.00/25g

135-19-3
754 suppliers
$9.00/1g

109-77-3
452 suppliers
$10.00/5g
![3-Amino-1H-benzo[f]chromene-2-carbonitrile](/CAS/20140525/GIF/CB32555561.gif)
141987-72-6
6 suppliers
inquiry